Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
(RULE 14A-101)
INFORMATION REQUIRED IN PROXY STATEMENT
PROXY STATEMENTSCHEDULE 14A INFORMATION
Proxy Statement Pursuant to Section 14(a) of
the Securities Exchange Act of 1934 (Amendment No. )
Filed by the Registrant x |
|
Filed by a Party other than the Registrant o |
|
Check the appropriate box: |
o | Preliminary Proxy Statement |
o | Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
x | Definitive Proxy Statement |
o | Definitive Additional Materials |
o | Soliciting Material under §240.14a-12 |
|
UNIQURE N.V. |
(Name of Registrant as Specified In Its Charter) |
|
|
(Name of Person(s) Filing Proxy Statement, if other than the Registrant) |
|
Payment of Filing Fee (Check the appropriate box): |
x | No fee required. |
o | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. |
| (1) | Title of each class of securities to which transaction applies: |
| | |
| (2) | Aggregate number of securities to which transaction applies: |
| | |
| (3) | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): |
| | |
| (4) | Proposed maximum aggregate value of transaction: |
| | |
| (5) | Total fee paid: |
| | |
o | Fee paid previously with preliminary materials. |
o | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
| (1) | Amount Previously Paid: |
| | |
| (2) | Form, Schedule or Registration Statement No.: |
| | |
| (3) | Filing Party: |
| | |
| (4) | Date Filed: |
| | |
| | | |
Table of Contents
uniQure N.V.
Paasheuvelweg 25a
1105BP Amsterdam
The Netherlands
+1-339-970-7000
NOTICE OF EXTRAORDINARY GENERAL MEETING OF SHAREHOLDERS
To be held on September 14, 2017
December 1, 2020
To the Shareholders of uniQure N.V.:
Notice is hereby given that anthe Extraordinary General Meeting of Shareholders (the “Extraordinary Meeting”) of uniQure N.V., a public company with limited liability (naamloze vennootschap) under the laws of the Netherlands (the “Company,” “uniQure,” and “we”), will be held on September 14, 2017,December 1, 2020, at 9:30 a.m.3:00 p.m., Central European Summer Time, atexclusively over the Company’s principal executive offices located at Paasheuvelweg 25a, 1105BP Amsterdam, the Netherlands,Internet via live audio webcast for the following purposes:
I. | Opening and announcements; |
II. | Appointment of Jeremy P. Springhorn,Robert Gut, M.D., Ph.D. as a non-executive director (voting proposal no.(Voting Proposal No. 1); |
III. | Appointment of Madhavan Balachandran as a non-executive director (voting proposal no. 2);
|
IV
| Any other business that may properly come before the meetingExtraordinary Meeting or any adjournment of the meeting;Extraordinary Meeting; and |
V.IV.
| Closing of the meeting.Extraordinary Meeting. |
Each person authorized to attend the Extraordinary Meeting may inspect the Agenda at the office of uniQure.
Our Board of Directors (our “Board”) recommends that you vote “FOR” each of the voting proposalsproposal noted above.
The record date is set atBoard has fixed the close of business Eastern Time on August 17, 2017 ESTNovember 3, 2020 as the record date and, therefore, only the Company’s shareholders of record (“Registered Shareholders”) at the close of business Eastern Time on August 17, 2017 ESTNovember 3, 2020 are entitled to receive this notice (this “Notice”) and to vote at the Extraordinary Meeting and any adjournment thereof.
Only shareholdersRegistered Shareholders who have given notice in writing to the Company by September 12, 2017November 30, 2020 of their intention to attend the Extraordinary Meeting in personover the Internet via live audio webcast are entitled to so attend the Extraordinary Meeting in person.Meeting. The conditions for attendance at the Extraordinary Meeting are as follows:follows. Registered Shareholders must:
1. Shareholders of record (“Registered Shareholders”) must (i)· notify the Company by November 30, 2020 of their intention to attend the Extraordinary Meeting over the Internet via the live audio webcast in accordance with the instructions as set out in the Proxy Statement (as defined below);
· vote their shares in advance of the Extraordinary Meeting by submittingInternet, by telephone or by mail in accordance with the instructions as set out in the Proxy Statement, and to ensure that their name and the number of registered shares held by them through the Company’s email address at investors@uniQure.comvotes are received no later than September 12, 2017 EST11:59 p.m. Central European Time on November 30, 2020;
· submit their questions regarding the agenda items in advance of the Extraordinary Meeting by email to investors@uniQure.com, and (ii) bring a form of personal picture identification to ensure that their questions are received no later than 11:59 p.m. Central European Time on November 30, 2020. The aim is to answer all questions so submitted, and there will be an opportunity to ask follow-up questions during the Extraordinary Meeting; and
2. Holders of shares held in street name (“Beneficial Holders”) must have their financial intermediary, agent or broker with whom the shares are on deposit issue a proxy to them which confirms they are authorized to take part in and vote at the Extraordinary Meeting. These Beneficial Holders must (i) notify the Company of their intention to attend the Extraordinary Meeting by submitting their name and the number of shares beneficially owned by them through the Company’s email address at investors@uniQure.com no later than close of business on September 12, 2017 EST, (ii) bring an account statement or a letter from the record holder indicating that you owned the shares as of the record date· listen to the Extraordinary Meeting (iii) bringover the proxy issued to them by their financial intermediary toInternet via the Extraordinary Meeting and (iv) bring a form of personal picture identification to the Extraordinary Meeting.live audio webcast at www.meetingcenter.io/277844738.
A proxy statement more fully describing the matters to be considered at the Extraordinary Meeting (the “Proxy Statement”) is attached to this Notice.
We have opted to provide our materials in connection with the Extraordinary Meeting pursuant to the full set delivery option in connection with the Extraordinary Meeting.option. Under the full set delivery option, a company delivers all proxy materials to its shareholders. The approximate date on which the Proxy Statement and Proxy Card are intended to be first sent or given to the Company’s shareholders (each a “Shareholder”, and collectively, the “Shareholders”) is August 18, 2017. This delivery can be by mail or, if a shareholder has previously agreed, by e-mail. Accordingly, you are receiving our proxy materials by mail or, if you previously agreed, by e-mail. These proxy materials include this Notice, the Proxy Statement, and the proxy card. In addition to delivering proxy materials to shareholders, the Companya company must also post all proxy materials on a publicly accessible website and provide information to shareholders about how to access that website. Accordingly, you should have received our proxy materials by mail or, if
Table of Contents
you previously agreed, by e-mail. These proxy materials include this Notice of Extraordinary General Meeting of Shareholders, Proxy Statement, proxy card. These materials are available free of charge athttp://www.edocumentview.com/QURE.
Your vote is important regardless of the number of uniQure ordinary shares that you own. If you do not plan on attending the Extraordinary Meeting and if you are a Registered Shareholder, please vote via the Internet or, if you are a Beneficial Holder, please submit the voting instruction form you receive from your broker or nominee as soon as possible so your shares can be voted at the meeting. You may submit your voting instruction form by mail. If you are a Registered Shareholder, you also may vote by telephone or by submitting a proxy card by mail. If you are a Beneficial Holder, you will receive instructions from your broker or other nominee explaining how to vote your shares, and you also may have the choice of instructing the record holder as to the voting of your shares over the Internet or by telephone. Follow the instructions on the voting instruction form you receive from your broker or nominee. You do not need to affix postage to the enclosed reply envelope if you mail it within the United States. If you attend the meeting, you may withdraw your proxy and vote your shares personally.
All proxies submitted to us will be tabulated by Computershare. All shares voted by Registered Shareholders present in person at the Extraordinary Meeting will be tabulated by the secretary designated by the chairmanChairman of the Extraordinary Meeting.
Table of Contents
All shareholders are extended an invitation to attend the Extraordinary Meeting.
| By Order of the Board of Directors, | |
| | |
| /s/ Matthew Kapusta | |
| Matthew Kapusta | |
| Chief Executive Officer, Chief Financial Officer and Executive Director | |
| August 15, 2017 November 2, 2020
| |
3
Table of Contents
Important Notice Regarding the Availability of Proxy Materials for the ShareholderExtraordinary General Meeting of Shareholders To Be Held on September 14, 2017December 1, 2020
The Proxy Statement and our Proxy Card are available at
http://www.edocumentview.com/QURE.QURE
and on our website at http://www.uniqure.com.
Table of Contents
NOTE REGARDING FORWARD-LOOKING STATEMENTS
Certain statements in the following proxy statement for the Extraordinary General Meeting of Shareholders are “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended or the Exchange Act,(the “Exchange Act”), and are subject to the safe harbor created by those sections. Forward-looking statements are based on our current assumptions, projections and expectations of future events, and are generally identified by words such as “may,” “will,” “should,” “could,” “would,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “predicts,” “potential” and similar expressions, or the negatives thereof, or future dates. Forward-looking statements involveare subject to risks and uncertainties that could cause actual results to differ materially from those projected or implied. The most significant factors known to us that could materially adversely affect our business, operations, industry, financial position or future financial performance are described in “Part I, Item 1A, Risk Factors” in our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission or SEC,(the “SEC”) on March 15, 2017,2, 2020 (the “Annual Report on Form 10-K”) and in “Part I, Item 1A, Risk Factors” andour most recent Quarterly Report on Form 10-Q filed with the SEC on August 8, 2017.October 27, 2020. You should not place undue reliance on any forward-looking statement, which speaks only as of the date made, and should recognize that forward-looking statements are predictions of future results, which may not occur as anticipated. Actual results could differ materially from those anticipated in the forward-looking statements and from historical results due to the risks and uncertainties described in our annual and quarterly reportsAnnual Report on Form 10-K, including in “Part I, Item 1A. Risk Factors,” as well as others that we may consider immaterial or do not anticipate at this time. The risks and uncertainties described in our annualAnnual Report on Form 10-K and quarterly reportsin our Quarterly Report on Form 10-Q are not exclusive and further information concerning our company and our businesses,business, including factors that potentially could materially affect our operating results or financial condition, may emerge from time to time. We undertake no obligation to update forward-looking statements to reflect actual results or changes in factors or assumptions affecting such forward-looking statements. We advise you, however, to consult any further disclosures we make on related subjects in our future Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K that we file with or furnish to the SEC.
1
Table of Contents
uniQure N.V.
Paasheuvelweg 25a
1105BP
1105 BP Amsterdam
The Netherlands
+1-339-970-7000
PROXY STATEMENT FOR THE EXTRAORDINARY GENERAL MEETING OF SHAREHOLDERS
To Be Held on September 14, 2017,December 1, 2020 at 9:30 a.m.3:00 p.m., Central European Summer Time
This proxy statement (the “Proxy Statement”), which includes the explanatory notes to the agenda for the Extraordinary General Meeting of Shareholders (the “Extraordinary Meeting”), and the accompanying proxy card (the “Proxy Card”), are being furnished with respect to the solicitation of proxies by the Board of Directors (the “Board”) of uniQure N.V., a public company with limited liability ((naamloze vennootschap) vennootschap) under the laws of the Netherlands (the “Company,” “uniQure” or “we”), for the Extraordinary Meeting. The Extraordinary Meeting will be held September 14, 2017, at 9:30 a.m CEST,3:00 p.m. Central European Time, on December 1, 2020, and at any adjournment thereof, atexclusively over the Company’s principal executive offices, Paasheuvelweg 25a, 1105BP Amsterdam, the Netherlands.Internet via live audio webcast.
The approximate date on which the Proxy Statement and Proxy Card are intended to be first being sent or given to the Company’s shareholders (each a “Shareholder”, and collectively, the “Shareholders”) is August 18, 2017.on or about the date hereof.
The purposes of the Extraordinary Meeting are to discuss andand/or vote on the following:
I. | Opening and announcements; |
II. | Appointment of Jeremy P. Springhorn,Robert Gut, M.D., Ph.D. as a non-executive director (voting proposal no.(Voting Proposal No. 1); |
III. | Appointment of Madhavan Balachandran as a non-executive director (voting proposal no. 2);
|
IV
| Any other business that may properly come before the meetingExtraordinary Meeting or any adjournment of the meeting;Extraordinary Meeting; and |
V.IV.
| Closing of the meeting.Extraordinary Meeting. |
Who May Vote
Shareholders of record of our ordinary shares (the “Ordinary Shares”) as ofat the close of business Eastern Time on August 17, 2017November 3, 2020 (the “Record Date”) are entitled to receive notice of and to vote at the Extraordinary Meeting and any adjournment thereof. On August 11, 2017,October 22, 2020, we had issued and outstanding 25,629,099 44,496,141 Ordinary Shares. We have no other securities entitled to vote at the Extraordinary Meeting. Each Ordinary Share is entitled to one vote on each matter. There is no cumulative voting.
A list of Shareholders entitled to vote at the Extraordinary Meeting will be available at the Extraordinary Meeting and will also be available for ten (10) days prior to the Extraordinary Meeting, during regular office hours, at the principal executive offices of the Company, located at Paasheuvelweg 25a, 1105BP1105 BP Amsterdam, the Netherlands, by contacting investor relations.Investor Relations at uniQure N.V., Paasheuvelweg 25a, 1105 BP Amsterdam, the Netherlands, by telephone at +1-339-970-7000, or by email to investors@uniQure.com.
Each matter proposed byUnder the Board shall be adopted by a simple majority of the votes cast at the Extraordinary Meeting. Under ourCompany’s Articles of Association and the Nasdaq rules, the presence at the Extraordinary Meeting of 33 1/3% of the outstanding Ordinary Shares, representedissued share capital, present in person or represented by proxy, is required for a quorum. “Abstentions“Abstentions” and “broker non-votes,” if any, will be counted as present and entitled to vote for purposes of determining whether a quorum is present for the transaction of business at the meeting.
“Broker non-votes” are shares represented at the Extraordinary Meeting held by brokers, bankers or other nominees (i.e., in “street name”) andthat are not voted on a particular proposal because the nominee does not have discretionary voting power with respect to that item and has not received instructions from the beneficial owner. Generally, brokerage firms may not vote to electappoint directors, because those proposals are considered “non-discretionary” items. Accordingly, if you do not instruct your broker how to vote your shares on “non-discretionary” matters, your broker will not be permitted to vote your shares on these matters. This is a “broker non- vote.”
2
Table of Contents
Methods of Voting
If you are a record holder of Ordinary Shares at the close of business Eastern Time on August 17, 2017,November 3, 2020, you may vote as follows:
· By Internet. Access the website of the Company’s tabulator, Computershare, at:
http://www.investorvote.com/QURE,using the voter control number printed on the furnished proxy card. Your shares will be voted in accordance with your instructions. You must specify how you want your shares voted or your Internet vote cannot be completed and you will receive an error message. If you vote on the Internet, you also may request electronic delivery of future proxy materials.
· By Telephone. Call 1-800-652-8683 toll-free from the U.S., U.S. territories and Canada and follow the instructions on the enclosed proxy card. Your shares will be voted in accordance with your instructions. You must specify how you want your shares voted or your telephone vote cannot be completed. You must have the control number that is included on the proxy card when voting.
· By Mail. Complete and mail a proxy card in the enclosed postage prepaid envelope to the address provided. Your proxyshares will be voted in accordance with your instructions. If you are mailed or otherwise receive or obtain a proxy card, and you choose to vote by telephone or by Internet, you do not have to return your proxy card.
·In Person at the Meeting. If you attend the Extraordinary Meeting over the Internet via live audio webcast, you will not be able to vote the shares you hold in real time over the Internet via live audio webcast, so please ensure that you vote in advance of the Extraordinary Meeting by Internet, by telephone or by mail, such in accordance with the above instructions. To be sure to bring a form of personal picture identification with you. You may deliverthat your completed proxy cardvote will be received in person, or you maytime (and no later than 11:59 p.m. Central European Time on November 30, 2020), please cast your vote by completing a ballot, which will beyour choice of available means at the meeting. Directions to the Annual Meeting are available by contacting Investor Relations at, uniQure N.V., Paasheuvelweg 25a, 1105BP Amsterdam, the Netherlands, telephone number +1-339-970-7000, email investors@uniQure.com.your earliest convenience.
If your Ordinary Shares are held in street name (held for your account by a broker or other nominee) at the close of business Eastern Time on August 17, 2017,November 3, 2020, you may vote:
· By Internet or By Telephone.You will receive instructions from your broker or other nominee if you are permitted to vote by internet or telephone.
· By Mail.You will receive instructions from your broker or other nominee explaining how to vote your shares.
·In Person at the Meeting.If you attend the meeting,Extraordinary Meeting over the Internet via live audio webcast, you will not be able to vote the shares you hold in addition to picture identification, you should bring an account statement or a letter fromstreet name in real time over the record holder indicatingInternet via live audio webcast, so please ensure that you owned the shares asvote in advance of the record date, and contactExtraordinary Meeting by Internet, by telephone or by mail, such in accordance with the broker or other nominee who holdsabove instructions. To be sure that your shares to obtain a broker’s proxy card and bring it with you to the meeting.vote will be received in time (and no later than 11:59 p.m. Central European Time on November 30, 2020), please cast your vote by your choice of available means at your earliest convenience.
Board’s Recommendations
The Board recommends a vote:
· Voting Proposal No. 1: “FOR” electionappointment of Jeremy P. Springhorn,Robert Gut, M.D., Ph.D. to the Board.
· Voting Proposal No. 2: “FOR” election of Madhavan Balachandran to the Board.as a non-executive director.
Voting by Proxy
The Ordinary Shares represented by any proxy duly given will be voted at the Extraordinary Meeting in accordance with the instructions of the Shareholder. You may vote “FOR” or “AGAINST” or “ABSTAIN” from each of the proposals.voting proposal. If no specific instructions are given, the shares will be voted “FOR” the voting proposalsproposal described in
3
Table of Contents
this Proxy Statement. In addition, if any other matters come before the Extraordinary Meeting, the persons named in the accompanying Proxy Card will vote in accordance with their best judgment with respect to such matters.
If we receive a signed and dated proxy card or receive your instructions by Internet or by telephone and your instructions do not specify how your shares are to be voted, your shares will be voted with the aforementioned Board’s recommendations.
Revoking Your Proxy
Even if you execute a proxy, you retain the right to revoke it and to change your vote by notifyingvote. You must notify us at any time beforeof your intention to revoke your proxy is voted.no later than 11:59 p.m. Central European Time on November 30, 2020. Such revocation may be effected in writing by execution of a subsequently dated proxy, or by a written notice of revocation, sent to the attention of Investor Relations at the address of our principal executive officeoffices set forth above, or by your attendance and voting in person at the Extraordinary Meeting or any adjournment thereof.above. Unless so revoked, the shares represented by a proxy, if received in time, will be voted in accordance with the directions given therein.
If the Extraordinary Meeting is postponed or adjourned for any reason, at any subsequent reconvening of the Extraordinary Meeting, all proxies will be voted in the same manner as the proxies would have been voted at the original convening of the Extraordinary Meeting (except for any proxies that have at that time effectively been revoked or withdrawn).
You are requested, regardless of the number of shares you own or your intention to attend the Extraordinary Meeting over the Internet via live audio webcast, to vote by proxy as soon as possible by proxy.possible. You do not need to affix postage to the enclosed reply envelope if you mail it within the United States.
Solicitation of Proxies
The expenses of solicitation of proxies will be paid by the Company. We may solicit proxies by mail, by electronic mail or by phone through agents of the Company. Additionally, the employees of the Company, who will receive no extra compensation therefor, may solicit proxies personally, by telephone, electronic mail, facsimile or mail. The Company will also reimburse banks, brokers or other institutions for their expenses incurred in sending proxies and proxy materials to the beneficial owners of shares held by them. The Company will not be making a solicitation through specially engaged employees or paid solicitors.
Delivery of Proxy Materials to Households
Only one copy of this Proxy Statement will be delivered to an address where two or more Shareholders reside unless we have received contrary instructions from a Shareholder residing at such address. AUpon written or oral request from a Shareholder, we will promptly deliver a separate copy of the Proxy Statement, Notice of Internet Availability of Proxy Materials, and Proxy Card will be delivered to each Shareholder at the shared address.
If you are a Shareholder who lives at a shared address and you would like additional copies of the Proxy Statement, or any future annual reports or proxy statements, please contact InvestorsInvestor Relations, uniQure N.V., Paasheuvelweg 25a, 1105BP1105 BP Amsterdam, the Netherlands, by telephone numberat +1-339-970-7000 or by email at investors@uniQure.com, and we will promptly mail you copies. This Proxy Statement is also available athttp://www.edocumentview.com/QURE. If you are receiving multiple copies of this Proxy Statement at your household and wish to receive only one, please contact Investor Relations at the mailing address, phone number or email address listed above.
Voting Results
The preliminary voting results will be announced at the Extraordinary Meeting. The final results will be disclosed in a Current Report on Form 8-K within four days after the meeting date.
4
Status as an “emerging growth company”
We are an “emerging growth company” as that term is used in the Jumpstart Our Business Startups (JOBS) ActTable of 2012 and, as such, have elected to comply with certain reduced public company reporting requirements. These reduced reporting requirements include reduced disclosure about our executive compensation arrangements and no non-binding advisory votes on executive compensation. We will remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of the completion of our initial public offering in February 2014, (b) in which we have total annual gross revenue of at least $1.07 billion, or (c) in which we are deemed to be a large accelerated filer, which means the market value of our ordinary shares that is held by non-affiliates exceeds $700 million as of the prior June 30th, and (2) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period.Contents
Contact for Additional Questions
If you hold your shares directly, please contact Investor Relations at uniQure N.V., Paasheuvelweg 25a, 1105BP1105 BP Amsterdam, the Netherlands, by telephone numberat +1-339-970-7000, or by email at investors@uniQure.com. If your shares are held in street name, please use the contact information provided on your voting instruction form or contact your broker or other nominee holder directly.
5
Table of Contents
AGENDA ITEM I—I — OPENING AND ANNOUNCEMENTS
The Chairman will open the Extraordinary Meeting and make any announcements.
AGENDA ITEMSITEM II and III— VOTING PROPOSAL NO. 1 - BOARD APPOINTMENT
VOTING PROPOSALS NO. 1 AND NO. 2— BOARD APPOINTMENTS
The Board is responsible for establishing broad corporate policies and monitoring the overall performance of the Company. It selects the Company’s senior management, delegates authority for the conduct of the Company’s day-to-day operations to those senior managers and monitors their performance. Members of the Board are kept informed of the Company’s business by, among other things, participating in Board and Committee meetings and by reviewing analyses and reports provided to them.
The Board is currently made up of seveneight directors. The termterms of office of twothree non-executive directors, Philip Astley-Sparke, David Meek, and Will Lewis, isPaula Soteropoulos, are scheduled to expire on the date of the 20182021 annual general meeting (the “2018 Annual Meeting”);of shareholders; the term of office of one executive director, Matthew Kapusta, is scheduled to expire on the date of the 20192022 annual general meeting (the “2019 Annual Meeting”);of shareholders; and the termterms of office of threefor four non-executive directors, Madhavan Balachandran, Jack Kaye, David Schaffer,Leonard Post, and Sander van Deventer, is scheduledJeremy Springhorn, are schedule to expire on the date of the 20202023 annual general meeting (the “2020 Annual Meeting”); and the term of officeshareholders. Under our Articles of one non-executive director, Paula Soteropoulos, is scheduled to expire on the date of the 2021 annual meeting (the “2021 Annual Meeting”). AllAssociation, all directors will hold office for a maximum term of four years, or until their earlier death, resignation, removalsuspension or disqualification.dismissal. However, the current practice of the Board is to nominate all directors, both executive and non-executive, for terms of office of three years. Our Board has implemented staggered terms to provide for a retirement schedule as required by our Articles of Association do not require the terms of the directors be staggered.
Mr. Lewis previously informed the Board that he anticipated that he would not be able to remain on the Board in the long term due to his other business commitments, and that if one or more additional nominees were identified by the Board for election, he would resign before the end of his term. Mr. Lewis has notified the Board that he will resign his position on the Board upon the election of one or both of the nominees at the Extraordinary Meeting.Association.
Dr. Sander van DeventerGut served as one of our executive directors since our extraordinary general meeting of shareholders in October 2018 until his resignation from his position as Chief Medical Officer in October 2020. The non-executive directors, following the recommendation of the Nominating and Mr. KapustaCorporate Governance Committee, have agreedmade a binding nomination to appoint Dr. Gut as a non-executive director in accordance with article 7.2 of our Articles of Association. The non-executive directors believe that he can best use his knowledgeDr. Gut’s unique experience makes him well qualified to continue serving on our Board. The non-executive directors also believe that Dr. Gut’s previous experience and experiencework as our Chief Medical Officer makes him well qualified to contributebe one of our non-executive directors.
The Board has nominated Robert Gut for appointment to the Company by workingBoard, to serve as a non-executive director until the Company’s chief scientific officer and resigning2022 annual general meeting of shareholders or until his position on the Board. The Board unanimously approved these changes, and as of August 7, 2017, Dr. van Deventer was appointed as the Company’s chief scientific officer. Dr. van Deventer has notified the Board that he will resign his position on the Board upon the election of oneearlier death, resignation, suspension or both of the nominees at the Extraordinary Meeting.dismissal.
The Board of Directors recommends a vote “FOR” the election of each of the nomineesnominee listed below.
The namesname and agesage as of the Record Date of the individualsindividual who areis our nomineesnominee for election as non-executive directors are:director is:
Name | | Age |
| Position |
|
Jeremy P. Springhorn,Robert Gut, M.D., Ph.D.
| | 5556
| | Director | |
ROBERT GUT, M.D., Ph.D. ROBERT GUT, M.D., Ph.D. Dr. Robert Gut, age 56, joined uniQure as our Chief Medical Officer in August 2018 and was elected as an executive director to our Board at the October 2018 extraordinary general meeting of shareholders. Dr. Gut was originally elected to the Board as a non-executive director in June 2018. He resigned that position in August 2018 to take the position of Chief Medical Officer because under Dutch law our non-executive directors are not able to hold executive positions with the Company. During that time, Dr. Gut led the clinical development, clinical operation, medical affairs, and patient advocacy teams that successfully initiated and executed our HOPE-B pivotal trial of etranacogene dezaparvovec for hemophilia B and our Phase 1/2 clinical trial of AMT-130 for the treatment of Huntington’s disease. Dr. Gut has more than 22 years of experience in the pharmaceutical and biopharmaceutical industry, leading clinical and medical activities in gene therapy, rare disorders, hematology, endocrinology, and other therapeutic areas. For the majority of his career, Dr. Gut worked at Novo Nordisk Inc. (NYSE: NVO), where he headed the company’s U.S. Biopharm Clinical & Medical organization with leading products in hemophilia, endocrinology, and women’s health (NovoSeven®, Norditropin®, and
6
Table of Contents
Vagifem®), totaling approximately $1.6 billion in U.S. revenue. Over his career, Dr. Gut’s contributions have helped achieve six FDA product approvals and three new product indications. Dr. Gut and his team have supported the launch of nine new products, overseeing clinical & medical activities including the creation of a new Medical Science Liaisons team and the Health Economics and Outcomes Research group. During his long industry career, he has also served as a member of the Advisory Committee for Reproductive Health Drugs and Drug Safety for the FDA’s CDER division, 2008-2012. Dr. Gut was the Chief Medical Officer of Versartis, Inc. in 2017-2018 and received his Doctor of Medicine degree from the Medical University of Lublin, and his Doctorate degree from Lublin Institute of Medicine, Poland. He attended numerous Executive Education Programs at Wharton, Stanford, and Harvard Business School.
On August 25, 2020, the Company entered into a separation agreement with Dr. Robert Gut (the “Gut Separation Agreement”) in connection with Dr. Gut’s retirement as an officer of the Company, effective as of October 14, 2020 (the “Gut Separation Date”).
The Gut Separation Agreement provides that Dr. Gut will receive, subject to the terms thereof, a lump sum severance payment equal to 140% of Dr. Gut’s annual base salary, or approximately $619,549, less applicable taxes and withholdings, within 30 days following the Separation Date, and reimbursement of full COBRA premiums until the earlier of (a) twelve (12) months from the Gut Separation Date, (b) the date on which Dr. Gut becomes eligible to participate in another employer’s group health plan, (c) the date on which Dr. Gut (or his eligible dependents, as applicable) is no longer eligible for COBRA coverage or (d) the date on which the Company in good faith determines that payments would result in a discriminatory health plan pursuant to the Patient Protection and Affordable Care Act of 2010. Dr. Gut will also be paid a pro-rata bonus equal to forty percent (40%) of his annual base salary. In addition, Dr. Gut will also be paid for any accrued but unused paid time off as of the Gut Separation Date.
Pursuant to the Gut Separation Agreement and except as noted above, Dr. Gut’s participation in all of the Company’s employee benefit plans will terminate as of the Gut Separation Date in accordance with the terms of those plans. Dr. Gut’s grants of equity in the Company shall be governed by the terms of the associated grant agreements, which are not altered by the terms of the Gut Separation Agreement.
The Gut Separation Agreement also provides that, through the earlier of the one-year anniversary of the Gut Separation Date or Dr. Gut’s appointment to the Board as a non-executive director, Dr. Gut will provide consulting services to the Company on an as-needed basis relating to the transition of the clinical operations organization. Dr. Gut will be reimbursed at a rate of $500 per hour.
Dr. Gut has also agreed to a customary release and is subject to certain confidentiality, disclosure, non-competition, and non-solicitation restrictions.
If appointed, the term of office for Dr. Gut will expire at the end of the 2022 annual general meeting of shareholders.
For information as to the Ordinary Shares held by Dr. Gut, see “Security Ownership of Certain Beneficial Owners and Management”.
As one our former executive directors and our Chief Medical Officer, the Board has determined that Dr. Gut will not be an independent director of the Company within the meaning of the director independence standards of the Nasdaq and SEC rules.
Other than as disclosed above regarding Dr. Gut’s compensation as consultant to the Company, there are no arrangements or understandings between the nominee, directors or executive officers and any other person pursuant to which our nominee, directors or executive officers have been selected for their respective positions.
VOTE REQUIRED
The resolution to appoint Dr. Gut shall be adopted, provided that the requisite quorum is present in person or represented by proxy at the Extraordinary Meeting, unless the Extraordinary Meeting resolves to overrule the binding nomination of the non-executive directors, which resolution requires at least a two-third majority of the votes cast at the Extraordinary Meeting, provided that such majority represents at least half of the issued share capital. Each Ordinary Share confers the right to cast one vote at the Extraordinary Meeting. Blank votes and invalid votes shall be regarded as not having been cast.
7
Table of Contents
THE BOARD UNANIMOUSLY RECOMMENDS THAT YOU VOTE “FOR” THE NOMINEE FOR DIRECTOR.
AGENDA ITEM III — ANY OTHER BUSINESS
The Extraordinary Meeting will review and discuss any other business brought to its attention.
AGENDA ITEM IV — CLOSING OF THE MEETING
The Chairman will adjourn the meeting.
8
Table of Contents
CORPORATE GOVERNANCE
Board of Directors (the “Board”) Leadership Structure and Composition
We have a one-tier board structure under Dutch law, meaning that executive and non-executive directors are members of the same board of directors. Our Articles of Association provide that the number of members of our Board will be determined by our Board, provided that the Board shall be comprised of at least one executive director and at least one non-executive director and provided further that the number of executive directors shall at all times be less than the number of non-executive directors. Our Board currently consists of eight directors, one of whom is an executive director and seven of whom are non-executive directors. If a director is to be appointed, the non-executive directors make a binding nomination, which is approved by the general meeting of shareholders pursuant to the procedure described in Voting Proposal Number 1. Under our Articles of Association, a general meeting of shareholders may suspend or dismiss a director by at least a two-thirds majority of votes cast, provided that such majority represents more than half of the issued share capital. The Board may suspend (but may not dismiss) an executive director. In the event of an absence or inability to act with respect to one or more of the directors, our Articles of Association provide that the non-executive directors shall be authorized to temporarily fill the vacant position for a period up to the first general meeting of shareholders, or in the case of a director unable to act, up to the moment he is no longer unable to act.
Under our Articles of Association and Dutch law, the members of our Board are collectively responsible for our management, general and financial affairs, and policy and strategy. Our executive directors are primarily responsible for managing our day-to-day affairs. Our non-executive directors supervise our executive directors and our general affairs, and provide general advice to them. In performing their duties, our directors are guided by the interest of our Company and, with the boundaries set by relevant Dutch law, must take into account the relevant interests of our stakeholders. In consultation with the Nominating and Corporate Governance Committee, the Board has determined that the current board structure is appropriate for the Company. Having staggered, multiple-year terms for our directors provides for stability, continuity and experience among our Board. Further, the Board believes that building a cohesive board of directors is an important goal. In our industry in particular, long-term focus is critical. The time horizon required for successful development of gene therapies makes it vital that we have a board of directors that understands the implications of this process and has the ability to develop and implement long-term strategies while benefiting from an in-depth knowledge of our business and operations. Our current board structure helps to ensure that there will be the continuity and stability of leadership required to resist the pressure to focus on short-term results at the expense of the long-term value and success of the Company. Our future success depends in significant part on the ability to attract and retain capable and experienced directors. In this regard, we believe that longer terms for our directors will enhance director independence from both management and stockholder special interest groups.
Under our Articles of Association and consistent with Dutch corporate governance principles, the Board appoints an executive director as Chief Executive Officer and appoints a non-executive director as Chairman of the Board. We believe that the separation of these roles serves our Shareholders and us well. Philip Astley-Sparke serves as our Chairman. The duties and responsibilities of the Chairman include, among others: determining the agenda and chairing the meetings of the Board, monitoring our Board to ensure that it operates effectively, ensuring that the directors receive accurate, timely, and clear information, encouraging active engagement by all directors, promoting effective relationships and open communication between the non-executive directors and the executive directors, and monitoring effective implementation of our Board decisions.
There are no arrangements or understandings between the directors or senior management and any other person pursuant to which our directors or senior management have been selected for their respective positions.
Directors and Senior Management
Set forth below are the names of our current directors and executive officers, their ages (as of November 3, 2020), all positions and offices that they hold with us, the period during which they have served as such, and their business experience during at least the last five years.
9
Table of Contents
Name |
| Age |
| Position |
Matthew Kapusta | | 48 | | Chief Executive Officer, Executive Director and Chief Financial Officer |
Philip Astley-Sparke | | 49 | | Chairman, Non-Executive Director |
Madhavan Balachandran | | 6669
| | Non-Executive Director |
Jack Kaye | | 76 | | Non-Executive Director |
David Meek | | 57 | | Non-Executive Director |
Leonard Post, Ph.D. | | 68 | | Non-Executive Director |
Paula Soteropoulos | | 52 | | Non-Executive Director |
Jeremy Springhorn, Ph.D. | | 58 | | Non-Executive Director |
Alexander Kuta, Ph.D. | | 60 | | Executive Vice President, Operations |
Ricardo Dolmetsch, , Ph.D. | | 51 | | Executive Vice President, Product and Research Development |
JEREMY P. SPRINGHORN, PH.D.MATTHEW KAPUSTA. Dr. Springhorn most recently served as Partner, Corporate Development at Flagship Pioneering from March 2015 until June 2017 where he worked with VentureLabs (in helping companies in various strategicMatthew Kapusta, age 48, has been Chief Executive Officer of uniQure since December 2016, and corporate development capacities and in creating next generation startups) and Flagship’s Corporate Partners. Prior to joining Flagship, Dr. Springhorn was one of the original scientists at Alexion Pharmaceuticals, where he played an integral role in its antibody engineering capabilities and was one of the original inventors of the drug Soliris. At Alexion Pharmaceuticals, Dr. Springhorn was Vice President of Corporate Strategy and Business Development from 2009 until March 2015 and head of global business development and corporate strategy from December 2006 until 2009. In 2006, Dr. Springhorn moved from research to business development, leveraging much of his drug development experience into the review of opportunities for ultra-orphan diseases. Along with building business development for Alexion, Dr. Springhorn also served as Head of Corporate Strategy as Alexion transitioned from a development firm to a global commercial stage company. Prior to 1992, Dr. Springhorn received his Ph.D. from Louisiana State University Medical Center in New Orleans and did his postdoctoral training at the Brigham and Woman’s Hospital in Boston. Dr Springhorn currently serves on the Company’s Board of OverseersDirectors. Mr. Kapusta also has served as our Chief Financial Officer since joining uniQure in January 2015. Prior to joining uniQure, Mr. Kapusta was Senior Vice President at AngioDynamics (NASDAQ: ANGO) from 2011 to 2014, responsible for Colby Collegecorporate development, strategic planning and national accounts. Prior to AngioDynamics, he served as Vice President, Finance and Strategic Planning and Analysis for Smith & Nephew Orthopaedics. Mr. Kapusta’s career also includes more than a decade of investment banking experience focused on emerging life sciences companies. Mr. Kapusta was Managing Director, Healthcare Investment Banking at Collins Stewart, and held various positions at Wells Fargo Securities, Robertson Stephens and PaineWebber. Mr. Kapusta holds a Master of Business Administration from New York University’s Stern School of Business, a Bachelor of Business Administration from University of Michigan’s Ross School of Business and earned his Certified Public Accountant license in 1996 while at Ernst & Young. We believe that Mr. Kapusta is qualified to serve as our CEO, Executive Director and Principal Financial Officer due to his broad expertise in the biotechnology and finance industries.
PHILIP ASTLEY-SPARKE. Philip Astley-Sparke, age 49, has served as a member of our Board since June 2015 and as chairman since 2016. He was previously president of uniQure Inc. from January 2013 until February 2015 and was responsible for building uniQure’s U.S. infrastructure. Mr. Astley-Sparke is currently Chief Executive Officer and co-founder of Replimune Group, Inc. (NASDAQ: REPL), a company developing second-generation oncolytic vaccines. Mr. Astley-Sparke served as Vice President and General Manager at Amgen, Inc. (NASDAQ: AMGEN), a biopharmaceutical company, until December 2011, following Amgen’s acquisition of BioVex Group, Inc., a biotechnology company, in March 2011. Mr. Astley-Sparke had been President and Chief Executive Officer of BioVex Group, which developed the scientific advisory boardfirst oncolytic vaccine to Arradial Inc.be approved in the western world following the approval of Imlygic in 2015. He oversaw the company’s relocation to the U.S. from the UK in 2005. Prior to BioVex, Mr. Astley- Sparke was a healthcare investment banker at Chase H&Q/Robert Fleming and qualified as wella Chartered Accountant with Arthur Andersen in London. Mr. Astley-Sparke has been a Venture Partner at Forbion Capital Partners, a venture capital fund, since May 2012. We believe that Mr. Astley-Sparke is qualified to serve as ona Non-Executive Director due to his expertise and experience in the Greater New Haven Chapter of the Juvenile Diabetes Research Foundation.biotechnology industry.
The Nominating and Corporate Governance Committee has recommended the appointment of Dr. Springhorn and the committee and Board believe that Dr. Springhorn would make a suitable board member because of his knowledge of the life sciences (including drug development) and of business development.
MADHAVAN BALACHANDRAN. Mr. Balachandran, age 69, has served as a member of our Board since September 2017. He joined Nutcracker Therapeutics, a private messenger RNA company, as COO in October 2020. Mr. Balachandran has been a director of Catalent (NYSE: CTLT) since May 2017. Mr. Balachandran was Executive Vice President, Operations of Amgen Inc., a global biotechnology company, from August 2012 until July 2016 and retired as an Executive Vice President in January 2017. Mr. Balachandran joined Amgen in 1997 as Associate Director, Engineering. He became Director, Engineering in 1998, and, from 1999 to 2001, he held the position of Senior Director, Engineering and Operations Services before moving to the position of Vice President, Information Systems from 2001 to 2002. Thereafter, Mr. Balachandran was Vice President, Puerto Rico Operations from May 2002 to February 2007. From February 2007 to October 2007, Mr. Balachandran was Vice President, Site Operations, and from October 2007 to August 2012, he held the position of Senior Vice President, Manufacturing. Prior to his tenure at Amgen, Mr. Balachandran held leadership positions at Copley Pharmaceuticals, now a part of Teva Pharmaceuticals Industries Ltd., and Burroughs WelcomeWellcome Company, a predecessor throughbefore mergers of GlaxoSmithKline plc. Mr. Balachandran holds a Master of Science degree in Chemical Engineering from The State
10
Table of Contents
University of New York at Buffalo and an MBA from East Carolina University.
The Nominating and Corporate Governance Committee has recommended the appointment of We believe Mr. Balachandran and the committee and Board believes that Mr. Balachandran would make a suitable board member because his knowledge of the industry and of the operations of life sciences companies.
Neither Dr. Springhorn nor Mr. Balachandran has previously served as a director or executive officer of the Company.
If elected, the term of office for Dr. Springhorn will expire on the date of the 2020 annual general meeting of shareholders and the term of office for Mr. Balachandran will expire on the date of the 2020 annual general meeting of shareholders.
Based upon information requested from and provided by each nominee for director concerning their background, employment and affiliations, including family relationships, our Board has determined that each of Dr. Springhorn and Mr. Balachandran has no relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and is independent within the meaning of the director independence standards of the Nasdaq rules and the SEC.
If the Extraordinary Meeting approves the appointment of Dr. Springhorn to the Board, the Board plans to appoint him to the Audit Committee and Nominating and Corporate Governance Committee. If the Extraordinary Meetings approves the appointment of Mr. Balachandran to the Board, the Board plans to appoint him to the Compensation Committee.
There are no arrangements or understandings between the nominees, directors or executive officers and any other person pursuant to which our nominees, directors or executive officers have been selected for their respective positions.
The Ordinary Shares held by each of Dr. Springhorn and Mr. Balachandran are included in “Security Ownership of Certain Beneficial Owners and Management.”
VOTE REQUIRED
All resolutions shall be adopted by at least a simple majority of the votes cast at the Extraordinary Meeting where more than one third of the issued share capital is represented. Each Ordinary Share confers the right to cast one vote at the Extraordinary Meeting. Blanc votes and invalid votes shall be regarded as not having been cast. Each proposed non-executive director appointment is considered a separate voting item under Dutch law.
THE BOARD UNANIMOUSLY RECOMMENDS THAT YOU VOTE “FOR” EACH OF THESE NOMINEES FOR DIRECTOR.
AGENDA ITEM IV—ANY OTHER BUSINESS
The Extraordinary Meeting will review and discuss any other business properly brought to its attention.
AGENDA ITEM V—CLOSING OF THE MEETING
The Chairman will adjourn the meeting.
CORPORATE GOVERNANCE
Board Leadership Structure and Composition
We have a one-tier board structure under Dutch law. Our Articles of Association provide that the number of members of our Board will be determined by our Board, provided that the Board shall be comprised of at least one executive director and at least two non-executive directors. Our Board currently consists of seven directors, one of whom is an executive director and six of whom are non-executive directors. If the Extraordinary Meeting approves the appointment of Dr. Springhorn and Mr. Balachandran (and assuming the resignation of Mr. Lewis and Dr. van Deventer), the Board will consist of seven directors, one of whom will be an executive director and six of whom will be non-executive directors.
If a director is to be appointed, the non-executive directors make a binding nomination which is approved by the general meeting of shareholders. Under our Articles of Association, a general meeting of shareholders may suspend or dismiss a director by at least a two thirds majority of votes cast, provided that such majority represents more than half the issued share capital. The Board may suspend (but may not dismiss) an executive director. In the event of an absence or inability to act with respect to one or more of the directors, our Articles of Association provide that the non-executive directors shall be authorized to temporarily fill the vacant position for a period up to the first general meeting, or in the case of a director unable to act, up to the moment he is no longer able to act.
Under our Articles of Association and Dutch law, the members of our Board are collectively responsible for our management, general and financial affairs, and policy and strategy. Our executive director is primarily responsible for managing our day-to-day affairs. Our non-executive directors supervise our executive director and our general affairs, and provide general advice to him. In performing their duties, our directors are guided by the interest of our company and, with the boundaries set by relevant Dutch law, must take into account the relevant interests of our stakeholders. In consultation with the Nominating and Corporate Governance Committee, the Board has determined that the current and proposed board structure is appropriate for the Company. Having multiple-year terms for each of our directors provides for stability, continuity and experience among our Board. Further, the Board believes that building a cohesive board of directors is an important goal. In our industry in particular, long-term focus is critical. The time horizon required for successful development of gene therapies makes it vital that we have a board that understands the implications of this process and has the ability to develop and implement long-term strategies while benefiting from an in-depth knowledge of our business and operations. Our current board structure helps to ensure that there will be the continuity and stability of leadership required to navigate a challenging environment while resisting the pressure to focus on short-term results at the expense of the long-term value and success of the Company. Our future success depends in significant part on the ability to attract and retain capable and experienced directors. In this regard, we believe that longer terms for our directors will enhance director independence from both management and shareholder special interest groups.
Under our Articles of Association and consistent with Dutch corporate governance principles, the Board appoints an executive director as chief executive officer and appoints a non-executive director as chairman of the Board. We believe that the separation of these roles serves our shareholders and us well. Philip Astley-Sparke serves as our Chairman. The duties and responsibilities of the Chairman include, among others, determining the agenda and chairing the meetings of the Board, managing our Board to ensure that it operates effectively, ensuring that the members of our Board receive accurate, timely, and clear information, encouraging active engagement by all members of our Board, promoting effective relationships and open communication between non-executive directors and the executive director, and monitoring effective implementation of our Board decisions.
There are no arrangements or understandings between the directors or executive officers and any other person pursuant to which our directors or executive officers have been selected for their respective positions.
Directors and Senior Management
Set forth below are the names of our current directors and officers, their ages (as of August 15, 2017), all positions and offices that they hold with us, the period during which they have served as such, and their business experience during at least the last five years.
Name
|
| Age
|
| Position
|
Matthew Kapusta
| | 45
| | Chief Executive Officer, interim Chief Financial Officer, Executive Director
|
Philip Astley-Sparke
| | 46
| | Chairman, Non-Executive Director
|
Jack Kaye
| | 73
| | Non-Executive Director
|
Will Lewis (1)
| | 48
| | Non-Executive Director
|
David Schaffer
| | 46
| | Non-Executive Director
|
Paula Soteropoulos
| | 49
| | Non-Executive Director
|
Sander van Deventer (2)
| | 62
| | Non-Executive Director; Chief Scientific Officer
|
Paul Firuta
| | 52
| | Chief Commercial Officer
|
Jonathan Garen
| | 51
| | Chief Business Officer
|
Maria Cantor
| | 49
| | Senior Vice President, Investor Relations & Communications
|
Alex Kuta
| | 58
| | Senior Vice President, Regulatory Affairs
|
Steven Zelenkofske
| | 58
| | Chief Medical Officer
|
Scott McMillan
| | 59
| | Chief Operating Officer
|
Christian Klemt
| | 44
| | Chief Accounting Officer
|
(1) Mr. Lewis has notified the Board that he will resign effective upon the Extraordinary Meeting and upon the election of one or both of the nominees at the Extraordinary Meeting.
(2) Dr. van Deventer has notified the Board that he will resign effective upon the Extraordinary Meeting and upon the election of one or both of the nominees at the Extraordinary Meeting.
MATTHEW KAPUSTA. Matthew Kapusta, age 45, joined uniQure as our chief financial officer in January 2015 and was elected to our Management Board at the 2015 annual general meeting. In December 2016 he was appointed our chief executive officer. Prior to joining uniQure, Mr. Kapusta was Senior Vice President at AngioDynamics (NASDAQ: ANGO) from 2011 to 2014, responsible for corporate development, strategic planning and national accounts. Prior to AngioDynamics, he served as Vice President, Finance for Smith & Nephew Orthopaedics. Mr. Kapusta’s career also includes more than a decade of investment banking experience focused on emerging life sciences companies. Mr. Kapusta was Managing Director, Healthcare Investment Banking at Collins Stewart, and held various positions at Wells Fargo Securities, Robertson Stephens and PaineWebber. Mr. Kapusta holds a Master of Business Administration from New York University’s Stern School of Business, a Bachelor of Business Administration from University of Michigan’s Ross School of Business and earned his Certified Public Accountant license in 1996 while at Ernst & Young. We believe that Mr. Kapusta is qualified to serve as our CEO, executive director and interim CFO due to his broad expertise in the biotechnology and finance industries.
PHILIP ASTLEY-SPARKE. Philip Astley-Sparke, age 46, has served as a member of our Board since June 2015 and as chairman since 2016. He was previously president of uniQure Inc. from January 2013 until February 2015 and was responsible for building uniQure’s U.S. infrastructure. Mr. Astley-Sparke is currently Executive Chairman and co-founder of Replimune Limited, a company developing second-generation oncolytic vaccines. Mr. Astley-Sparke served as vice president and general manager at Amgen, Inc. (NASDAQ: AMGEN), a biopharmaceutical company, until December 2011, following Amgen’s acquisition of BioVex Group, Inc., a biotechnology company, in March 2011. Mr. Astley-Sparke had been President and Chief Executive Officer of BioVex Group, which developed the first oncolytic vaccine to be approved in the western world following the approval of Imlygic in 2015. He oversaw the company’s relocation to the U.S. from the UK in 2005. Prior to BioVex, Mr. Astley-Sparke was a healthcare investment banker at Chase H&Q/Robert Fleming and qualified as a Chartered Accountant with Arthur Andersen in London. Mr Astley-Sparke has been a venture partner at Forbion Capital Partners, a venture capital fund, since May 2012 and serves as Chairman of the Board of Oxyrane, a biotechnology company. We believe that Mr. Astley-Sparke is qualified to serve as a non-executive directorNon-Executive Director due to his expertise andextensive experience in the biotechnology industry.
JACK KAYE.Jack Kaye, age 73,76, has served as a member of our Board since 2016. Mr. Kaye has also served as Chairman of the Audit Committee of Keryx Biopharmaceuticals, Inc. (NASDAQ: KERX) from September 2006 to May 2016 and is currently chairman of the Audit Committee and a member of the Compensation Committee of Dyadic International, Inc. (OTC: DYAI). Mr. Kaye began his career at Deloitte LLP, an international accounting, tax and consulting firm, in 1970, and was a partner in the firm from 19701978 until May 2006. At Deloitte, he was responsible for servicing a diverse client base of public and private, global and domestic companies in a variety of industries. Mr. Kaye has extensive experience consulting with clients on accounting and reporting matters, private and public debt financings, SEC rules and regulations and corporate governance/Sarbanes-Oxley matters. Prior to retiring, Mr. Kaye served as Partner-in-ChargePartner- in-Charge of Deloitte’s Tri-State Core Client practice, a position he held for more than 20 years. Mr. Kaye has a Bachelor of Business Administration from Baruch College and is a Certified Public Accountant. We believe that Mr. Kaye is qualified to serve as a non-executive directorNon- Executive Director due to his extensive accounting and financial experience.
WILL LEWIS.DAVID MEEK. Will Lewis,David Meek, age 48,57, has served as30 years of experience in the pharmaceutical industry, where he has held various global executive positions in major pharmaceutical and biotechnology companies. In January 2020, Mr. Meek was appointed President & CEO of FerGene, a member of our Board since June 2014.gene therapy biotech focused on cancer. From July 2016 to January 2020, Mr. Lewis is currently President, Chief Executive OfficerMeek was CEO and a member of the Board of Directors of Insmed (NASDAQ: INSM), a biopharmaceutical company specialized in inhalation therapies for orphan lung diseases.Ipsen. Prior to joining Insmed in 2012,Ipsen, he was PresidentExecutive Vice-President and Chief Financial Officer of Aegerion Pharmaceuticals, Inc. (NASDAQ: AEGR), which he also co-founded. At Aegerion, he played a pivotal role in reorienting the company’s strategy to focus on orphan disease indications. He previously worked in the U.S. and Europe in investment banking for JP Morgan, Robertson Stephens and Wells Fargo. During his time in banking, he was involved in a broad range of domestic and international capital raises and advisory work valued at more than $20 billion. He serves on the Board of Directors of Oberlin College and is a memberPresident of the Visiting Committeesoncology division of Baxalta prior to the Weatherhead Schoolacquisition of ManagementBaxalta by Shire. He spent 2 years as CCO of Case Western Reserve UniversityEndocyte. Mr. Meek also spent 8 years at Novartis as a global franchise head, CEO of Novartis Canada, and The Hawken School.region head of oncology for northern, central and Eastern Europe. He also spent 14 years at Johnson & Johnson and Janssen Pharmaceutica, where he held a variety of senior U.S. sales and marketing positions. Mr. Meek holds a B.A. from Oberlin College and an M.B.A./J.D. from Case Western Reserve University. We believe that
Mr. Lewis is qualified to serve as a non-executive director due to the depthUniversity of his experience in the biotechnology and finance industries. Mr. Lewis has communicated to the Board that he will resign his position on the Board upon the election of both of the nominees at the Extraordinary Meeting.Cincinnati.
DAVID SCHAFFER.LEONARD POST, PH.D. David Schaffer,Dr. Post, age 46,68, has served as a member of our Board since January 2014. Dr. Schaffer is Professor of Chemical and Biomolecular Engineering, Bioengineering, and Neuroscience at University of California Berkeley, a position he has held since 2007, as well as Director of the Berkeley Stem Cell Center since 2011. Dr. Schaffer is also co-founder of 4D Molecular Therapeutics, a company specializing proprietary technology for gene therapy products. We entered into a collaboration and license agreement with 4D Molecular Therapeutics in January 2014. Previously, Dr. Schaffer was Assistant Professor from 1999 to 2005 and Associate Professor from 2005 to 2007 at the University of California, Berkeley Department of Chemical Engineering & Helen Wills Neuroscience Institute. He has served on the boards of the American Society for Gene and Cell Therapy and the Society for Biological Engineering. He has more than 20over 35 years of experience in chemicalthe pharmaceutical industry where he has held various global executive positions and molecular engineering,has extensive experience in research and stem celldevelopment of product candidates. Since July 2016, Dr. Post has served as Chief Scientific Officer of Vivace Therapeutics, an oncology company working on small molecules targeting the hippo pathway, and is also Chief Scientific Officer of its sister company Virtuoso Therapeutics, a company working on bispecific antibodies for oncology. From February 2010 until June 2016, Dr. Post worked at BioMarin (NASDAQ: BMRN), in various positions including Chief Scientific Officer. During that time he oversaw the initiation of BioMarin’s first gene therapy research,project for hemophilia A. Prior to that, Dr. Post served as Chief Scientific Officer of LEAD Therapeutics, Senior Vice President of Research & Development at Onyx Pharmaceuticals, and Vice President of Discovery Research at Parke-Davis Pharmaceuticals. He is also currently an advisor to Canaan Partners. Dr. Post is a virologist by training and did early work on engineering of herpes simplex virus as a postdoctoral fellow. He has over 165 scientific publications, and serves on five journal editorial boards and five industrial scientific advisory boards. Dr. Schaffer holds a bachelorBachelor of scienceScience degree in Chemical EngineeringChemistry from Stanfordthe University of Michigan, and a Ph.D.Doctorate degree in Chemical EngineeringBiochemistry from the Massachusetts InstituteUniversity of Technology. We believe that Dr. Schaffer is qualified to serve as a non-executive director due to his experience in the biotechnology industry and his expertise in that field.Wisconsin.
PAULA SOTEROPOULOS.Paula Soteropoulos, age 49,52, has served as a member of our Board since July 2013. Ms. Soteropoulos is presidentan executive leader with more than 30 years of experience in the biopharma industry in areas of drug development, manufacturing, business development, global commercialization and chief executive officercompany building. Since March 2020, she serves as Strategic Advisor to 5AM Ventures and is Executive Chairman of a 5AM Venture NewCo. From January 2015 through September 2019, she served as President and Chief Executive Officer of Akcea Therapeutics a position she has held since January 2015.(NASDAQ: AKCA). From July 2013 to December 2014, she served as Senior Vice President and General Manager, Cardiometabolic Business and Strategic Alliances at Moderna Therapeutics Inc. Prior to this, Ms. Soteropoulos worked at Genzyme Corporation, a biotechnology company, from 1992 to 2013, most recently as Vice President and General Manager, Cardiovascular, Rare Diseases. Ms. Soteropoulos holds a bachelorBachelor of scienceScience degree in chemical engineering and a masterMaster of scienceScience degree in chemical and biochemical engineering, both from Tufts University, and holds an executive management certificate from the University of Virginia, Darden Graduate School of Business Administration. Ms. Soteropoulos serves on the Advisory Board for the Chemical and Biological Engineering Department of Tufts University. We believe Ms. Soteropoulos is qualified to serve as a non-executive directorNon-Executive Director due to her extensive experience in the biotechnology industry.
DR. SANDER VAN DEVENTER.JEREMY SPRINGHORN, PH.D. Dr. Sander van Deventer,Springhorn, age 62,58, has served as a member of our Board since April 2012 andSeptember 2017. Since November 2017, Dr. Springhorn has been Chief Business Officer of Syros Pharmaceuticals (NASDAQ:
11
Table of Contents
SYRS), Inc. Prior to taking his position at Syros, Dr. Springhorn served as member of the AMT supervisory boardPartner, Corporate Development at Flagship Pioneering from April 2010March 2015 until June 2017 where he worked with VentureLabs in helping companies in various strategic and corporate development capacities and in creating next generation startups and with Flagship’s Corporate Limited Partners. Prior to April 2012.joining Flagship, Dr. van DeventerSpringhorn was one of our co-founders. Hethe original scientists at Alexion Pharmaceuticals, Inc. (NASDAQ: ALXN), where he played an integral role in its antibody engineering capabilities and was one of the original inventors of the drug Soliris®. At Alexion Pharmaceuticals, Dr. Springhorn was Vice President of Corporate Strategy and Business Development from 2006 until March 2015. Dr. Springhorn started at Alexion in 1992 where he served as our interim Chief Executive Officerin various leadership roles in R&D before switching to Business Development in 2006. Prior to 1992, Dr. Springhorn received his Ph.D. from February to October 2009. He has been Professor of Translational Gastroenterology at the LeidenLouisiana State University Medical Center since 2008in New Orleans and is a partner of Forbion Capital Partners, which he joined in 2006. Hehis BA from Colby College. Dr. Springhorn currently serves on the boardsBoard of enGene Inc., Argos Biotherapeutics, gICareDirectors for NMD Pharma, IncBoard of Advisors for Mythic Therapeutics and Hookipa Biotech. He was previously a professor, headthe Board of the department of experimental medicine and chairman of the department of gastroenterology of the Academic Medical Center at the University of Amsterdam from 2002 to 2004, and subsequently professor of experimental medicine at the University of Amsterdam Medical School until 2008. Dr. van Deventer is currently a professor at Leiden University Medical Center. He has more than 15 years of experience in biotechnology product development. He is the author of more than 400 scientific articles in peer-reviewed journals, and he serves as an advisor to regulatory authorities including the EMA and FDA. Dr. van Deventer holds a degree in medicine as well as a Ph.D. from the University of Amsterdam.Visitors for Colby College. We believe that Dr. van DeventerSpringhorn is qualified to serve as non-executive directora Non-Executive Director due to his expertiseextensive experience in the biotechnology industry and his service on the boards of directors of other biotechnology companies. As of August 7, 2017, Dr. van Deventer joined the Company’s management team as its chief scientific officer. He has communicated to the Board that he will resign his position on the Board upon the election of one or both of the nominees at the Extraordinary Meeting.industry.
PAUL FIRUTA.ALEXANDER KUTA, PH.D. Paul Firuta,Dr. Kuta, age 52,60, joined uniQure in January 2017 and has served as our Chief Commercial Officer since May 2016. Mr. Firuta has more than 25 years of industry experience successfully commercializing products targeting rare diseases. He joined from BioBlast Pharma (NASDAQ: ORPN) where he held the position of Chief Commercial Officer from August 2015 to April 2016. Prior to this, from March 2014 to February 2015 he was President of U.S. Commercial Operations at NPS Pharmaceuticals (NASDAQ: NPSP) were he lead the development and execution of the Gattex® sales and marketing plan as well as the commercial launch of Natpara®. Over the course of his career, Mr. Firuta has held various leadership roles at biopharmaceutical companies includingExecutive Vice President, and General Manager, Americas at ViroPharma, Inc. (NASDAQ: VPHM) where he was responsible for U.S. commercial operations for Cinryze® and Vancocin®, representing over $400 million in U.S. revenue. Additionally, he was Vice President — Managed Markets at LEV Pharmaceuticals and Executive Director National Accounts at OraPharma. Mr. Firuta holds a Master of Business Administration from St. Joseph’s University in Philadelphia, Pennsylvania and a Bachelor of Science degree from King’s College, Wilkes-Barre, Pennsylvania.
JONATHAN GAREN. Jonathan Garen, age 51, has served as our Chief Business OfficerOperations since July 2016. Most recently, from 2015 to 2016, Mr. Garen served as Chief Business Officer at Syros Pharmaceuticals (NASDAQ: SYRS), where he was responsible for business transactions including partnering Syros’ technology platform and drug assets, and bringing in products to enhance and accelerate its pipeline.August 2019. Prior to joining Syros, Mr. GarenuniQure, he was the Assistant Vice President of Business Development at Forest Laboratories
(NASDAQ: FRX) from 2003 to 2014, and subsequently, Actavis, plc until 2015 following its acquisition of Forest Laboratories. At Forest Laboratories and Actavis, Mr. Garen was responsible for numerous acquisitions and license agreements to build the companies’ pipeline in all its focus therapeutic areas, and led a team of business development professionals. Earlier in his career, Mr. Garen was Director of Global Licensing with Pharmacia Corporation and a Founder and Vice President of Technology Exchange, Inc., in New York, NY. Mr. Garen holds a Master of Environmental Science degree from Yale University and a Bachelor of Science degree in Physics from the Massachusetts Institute of Technology.
MARIA CANTOR. Maria Cantor, age 49, has served as our Senior Vice President of Investor Relations and Communications since June 2016. Ms. Cantor joined uniQure from ARIAD Pharmaceuticals, Inc. (NASDAQ: ARIA) where she most recently held the role of Senior Vice President, Corporate Affairs. She was a member of the senior management team and was responsible for investor relations and corporate communications for the company since 2008. She played an active role in several successful financing rounds for ARIAD and led the communications program for the clinical development, regulatory approval and product launch of the company’s first cancer therapeutic, Iclusig®, approved in both the U.S. and Europe. Prior to joining ARIAD, she held the position of Senior Director, Corporate Communications at Genzyme, where she was involved in all aspects of corporate and product communications from 2001 to 2008. In addition to her extensive expertise as a communicator for leading biotechnology companies, Ms. Cantor also held senior communications positions within the healthcare industry including Director, Marketing and Public Relations at the St. Elizabeth Medical Center in Boston, and as Director, Marketing and Communications at Optima Healthcare Inc. in Manchester, New Hampshire. Ms. Cantor holds a Master of Science in Communications Management from Syracuse University, S.I. Newhouse School of Public Communications in Syracuse, New York and a Bachelor of Science in Mass Communication/Broadcast Journalism from Emerson College in Boston.
ALEX KUTA. Alex Kuta, age 58, has served as our Senior Vice President Regulatory Affairs since January 2017. Dr. Kuta joined uniQure from EMD Serono where he served as Vice President of Research & Development Global Regulatory Affairs from 2016 to 2017 and where he was a member of the U.S. Leadership Team. In this role, he wasfor EMD Serono, responsible for driving the strategic direction of EMD Serono’s regulatory efforts in immune-mediated diseases, oncology and biologics regulatory CMC, as well as strengthening interactions with the U.S. Food and Drug Administration (FDA). Priorfrom January 2016 to joiningSeptember 2016. He joined EMD Serono in April 2013 as Vice President, Head of US Regulatory Affairs; while at EMD Serono he served on the US Leadership Team. From April 2012 to March 2013, Dr. Kuta was Vice President of Global Regulatory Affairs and a member of the Executive Leadership Team at Lantheus Medical Imaging from 2012 to 2013.Imaging. His previous industry experience includes senior regulatory leadership roles at AMAG Pharmaceuticals (NASDAQ: AMAG) and atfrom August 2010 to April 2012 as well as Genzyme Corporation from August 1995 to July 2010 where he served for 15 yearsworked in regulatory leadership positionsthe areas of increasing responsibility.rare diseases, cell and gene therapy, therapeutic proteins and biomaterials. Prior to joining industry, he was Chief of the Cytokine and Gene Therapy Branch in the Center for Biologics at FDA.FDA from January 1993 to August 1995 and a Scientific Reviewer from January 1990 to January 1993. Dr. Kuta has also served as a member ofon the BIO Regulatory Affairs Leadership Committee - Cell and Gene Therapy Working Group, andas reviewer for the National Gene Vector Laboratories program, on the ICH (M6) Gene Therapy Working Group.Group and is currently on the scientific review board of the Gene Therapy Resource Program of NHLBI/NIH. Dr. Kuta holds a Bachelor of Science degree from Saint John’s University, Collegeville, MN and a Ph.D. from the Chicago Medical School at Rosalind Franklin U-Med & Science. He conducted his post-doctoral studies at the National Cancer Institute/National Institutes of Health.
STEVEN ZELENKOFSKE.RICARDO DOLMETSCH, Ph.D. Stephen Zelenkofske,Dr. Ricardo Dolmetsch, age 58, has served51, joined uniQure in September 2020 as our Chief Medical Officer since June 2017.President, Research & Development. He is responsible for uniQure’s gene therapy research activities, as well as nonclinical development, process development, analytical development and vector development. Prior to joining uniQure, Dr. Zelenkofske served as Vice President and Therapeutic Area HeadDolmetsch was employed by Novartis Institutes for Biomedical Research (NIBR), the research arm of Cardiovascular/Metabolism for AstraZenecaNovartis AG (NYSE: AZN) from November 2014. Prior to joining Astra Zeneca, from January 2009 to November 2014NVS), where he served as Chiefthe Global Head of Neuroscience since 2013. There, he led the development of treatments for neurodegenerative and neuropsychiatric diseases, including rare genetically defined disorders. During his tenure, Dr. Dolmetsch played an instrumental role in Novartis’ acquisition of AveXis in 2018 and the successful approval of Zolgensma, a commercial gene therapy for patients with spinal muscular atrophy Type 1. Prior to NIBR, Dr. Dolmetsch was a Professor at Stanford Medical OfficerSchool and a Senior Director at the Allen Institute for Regado Biosciences, a developer of RNA aptamer therapies. Dr. Zelenkofske has nearly 15 years of clinical research experience within the industry, having previously held leadership positions at AstraZeneca, Sanofi-Aventis (NYSE: SNY), Boston Scientific (NYSE: BSX), and Novartis Pharmaceuticals (NYSE: NVS). His clinical experience outside of industry includes serving on the cardiology and electrophysiology teamsBrain Science in Seattle, Washington. He obtained his bachelor’s degree with Lehigh Valley Heart Specialists, Heart Care Group and Cardiology Care Specialists, allhonors from Brown University, earned his Ph.D. in Allentown, PA, and on the medical staff of Episcopal Hospital in Philadelphia. Dr. Zelenkofske holds Bachelor of Science and Master of Science degreesneuroscience from EmoryStanford University and a Doctor of Osteopathic Medicine degree from the Philadelphia College of Osteopathic Medicine. He conducted his graduate medical educationpostdoctoral training at the Philadelphia College of Osteopathic MedicineHarvard University Medical School and is board-certified in internal medicine, cardiology and cardiac electrophysiology.Children’s Hospital Boston.
SCOTT MCMILLAN. Scott McMillan, age 59, has served as our Chief Operating Officer since August 2017. Prior to joining uniQure, Dr. McMillan served as Senior Vice President, Quality & Technical Operations for AMAG Pharmaceuticals Inc. (“AMAG”) (NASDAQ: AMAG), a commercial stage biopharmaceutical company, from 2008 to 2017. Prior to AMAG, Dr. McMillan spent four years at AVANT Immunotherapeutics, Inc. (“AVANT”), a biopharmaceutical company, from 2004 to 2008, where he was promoted to Sr. Director - Manufacturing Operations & Process Development in 2007. From 2007 to 2017, Dr. McMillan has been a member of the Industrial Advisory Board of the Department of Chemical Engineering at Northeastern. Dr. McMillan holds a Ph.D. in Chemical Engineering from the Georgia Institute of Technology and a B.S. in Chemical Engineering from the University of Delaware.
CHRISTIAN KLEMT. Christian Klemt, age 44, has served as our Chief Accounting Officer since August 2017. Prior to being appointed our Chief Accounting Officer, he served as our Global Controller from September 2015. Prior to joining uniQure, Mr. Klemt was Regional Finance Director as well as Managing Director and Country Manager for the Netherlands at CGG from 2009 to 2015. Prior to CGG, he served in various finance roles including Group Finance Manager with LyondellBasell. Mr. Klemt holds a Master of Business Administration from the University of Muenster, Germany. He qualified as a German Certified Public Accountant and Tax
Advisor in 2005 while at KPMG.
Risk Oversight
Generally, the Board, in its advisory capacity, and the Company’s management regularly review the Company’s strategic plan which includes, among other things, the various business, clinical, developmental, financial and other market risks confronting, and opportunities available to, the Company at any given time. Specifically, pursuant to the Company’s Corporate Governance Guidelines and Board Rules, available on the Company’s website www.uniqure.com, the Board is charged with assessing major risks facing the Company and reviewing options to mitigate such risks.
The Board performs this oversight role by using several different levels of review. In connection with its reviews of the operations and corporate functions of the Company, the Board addresses the primary risks associated with those
12
Table of Contents
operations and corporate functions. In addition, the Board reviews the risks associated with the Company’s business strategies periodically throughout the year as part of its consideration of undertaking any such business strategies.
The Board has delegated certain risk oversight responsibilities to its committees (the “Committees”). Each of our Board’s Committees also oversees the management of the Company’s risk that falls within each Committee’s areas of responsibility. In performing this function, each Committee has full access to management, as well as the ability to engage advisors. For example, the Audit Committee is required to regularly review and discuss with management the Company’s major financial risk exposures and the steps management has taken to monitor and control such exposures. The Nominating and Corporate Governance Committee is required to regularly review the corporate governance principles of the Company and recommend to the Board any proposed changes it may deem appropriate. The Compensation Committee considers risks related to the attraction and retention of professional talent and the implementation and administration of compensation and benefit plans affecting the Company’s employees. The Research and Development Committee reviews our technology, research and development activities, product pipeline, and manufacturing platform to advise our management team and the Board on the Company’s technology. All Committees are required, pursuant to their respective charters, to report regularly to the Board. The activities of the Audit, Nominating and Corporate Governance and Compensation Committees are more fully described below.
Board Determination of Director Independence
Our securities are listed on the NASDAQNasdaq Global Select Market (“Nasdaq”) and we use the standards of “independence” prescribed by rules set forth by Nasdaq. Under Nasdaq rules, a majority of a listed company’s board of directors must be comprised of independent directors. In addition, Nasdaq rules require that, subject to specified exceptions, each member of a listed company’s audit committee and compensation committee be independent and, in the case of audit committees, satisfy additional independence criteria set forth in Rule 10A-3, under the Securities Exchange Act of 1934, as amended, (the “Exchange Act”).Act. Under Nasdaq rules, a director will only qualify as an “independent director” if, in the opinion of that company’s board of directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director.
Based upon information requested from and provided by each director concerning their background, employment and affiliations, including family relationships, our Board has determined that each of Philip-Astley Sparke, Madhavan Balachandran, Jack Kaye, Will Lewis,David Meek, Paula Soteropoulos, Dr. Jeremy Springhorn and Madhavan Balachandran hasLeonard Post have no relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and is independent within the meaning of the director independence standards of the Nasdaq rules and the SEC. Our Board has determined that each of Matthew Kapusta Dr. Sander van Deventer, David Schaffer and Philip Astley-Sparke dodoes not qualify as “independent” under the Nasdaq rules. Our Board has also determined that each of the current members of our Audit Committee and our Compensation Committee satisfies the independence standards for such committee established by Rule 10A-3 under the Exchange Act, the SEC rules and the Nasdaq rules, as applicable, and that the current members of the Nominating and Corporate Governance Committee and the Research and Development Committee are also independent. In making these determinations, the directors reviewed and discussed information provided by the directors and the Company with regard to each director’s business and personal activities as they may relate to the Company and the Company’s management.
Board Meetings
The Board met 611 times during the calendar year ended December 31, 2016. Other than Joseph Feczko, who is no longer a director, each2019. Each of theour directors attended at least 75% of the meetings of the Board and the Committees on which he or she served during the year ended December 31, 20162019 (in each case, which was held during the period for which he or she was a director and/or a member of the applicable Committee). Ferdinand Verdonck, the chairman at the time,Mr. Astley-Sparke, Mr. Balachandran, Dr. Gut, Mr. Kapusta, Mr. Meek, and Dr. van DeventerMs. Soteropoulos attended our 2016 annual meeting2019 Annual General Meeting of shareholdersShareholders held on June 15, 2016.19, 2019. The Company encourages its directors to attend the annual general meeting of Shareholders.shareholders. Executive sessions, or meetings of the independent directors without management present, are held regularly.
Committees and Committee Meetings
The Board has a standing Audit Committee, Nominating and Corporate Governance Committee, Compensation Committee, and CompensationResearch and Development Committee, each of which is comprised solely of independent directors,
13
Table of Contents
and is described more fully below. The members of each Committee are appointed
by our Board. From time to time, the Board may establish other committees. Below is a description of the threefour principal Committees.Committees of our Board.
Audit Committee and Audit Committee Financial Expert
The Audit Committee is currently comprised of Jack Kaye, Paula SoteropoulosPhilip Astley-Sparke and Philip Astley-Sparke. JackJeremy Springhorn. Mr. Kaye serves as chairthe Chair of the Audit Committee. The Audit Committee andhas determined that Mr. Kaye is the Audit Committee’san “audit committee financial expert” within the meaning of the SEC’s rules and regulations. Inregulations and has the eventlevel of their successful elections at the Extraordinary Meeting, the Board anticipates that Dr. Springhorn will be appointed to the Audit Committee and that Ms. Soteropoulos will resign.financial sophistication required by Nasdaq Rule 5605(c)(2) (A). Each of JackMr. Kaye, Paula SoteropoulosMr. Astley-Sparke and Dr. Springhorn satisfies the director independence standards and the independence standards for members of the Audit Committee established by SEC and Nasdaq. Mr. Astley-Sparke meets the criteria for appointment to the Audit Committee pursuant to the “exceptional and limited circumstances” set out in Nasdaq listing rule 5605(c)(2)(B). Pursuant to such exception, a director who (i) is not an “independent director” as defined by Nasdaq rules, (ii) meets the criteria set forth in Section 10A(m)(3) of the Exchange Act and the rules thereunder, and (iii) is not currently an executive officer or employee or a family member of an executive officer, may be appointed to a company’s audit committee, if a company’s board, under exceptional and limited circumstances, determines that the membership on the audit committee by such individual is required by the best interests of the Company and its shareholders.
As noted above, our Board determined that Philip Astley-Sparke does not currently qualify as “independent” under Nasdaq rules, because he served as President of uniQure, Inc., our US subsidiary, from January 2013 until February 2015. Our Board determined that it was in the best interests of the Company and its shareholders to appoint Mr. Astley-Sparke to the Audit Committee following the 2017 Annual General Meeting of Shareholders. Mr. Astley-Sparke was responsible for building our Company’s U.S. infrastructure and has had in-depth experience with several biotechnology companies, and therefore has a deep understanding of our Company and our industry. In addition, Mr. Astley-Sparke’s experience as an investment banker and chartered accountant has given him a high degree of financial sophistication and understanding of financial statements, which make him a valuable member of the Audit Committee. Our Board reviewed Mr. Astley-Sparke’s business relationship with the Company that disqualifies him as “independent” under the Nasdaq Rules and concluded that this relationship did not impair his ability to fulfill his responsibilities as a member of the Audit Committee. In accordance with Rule 5605(c)(2)(B), Mr. Astley-Sparke has not served on the Audit Committee for more than two years and has not chaired the Audit Committee.
As noted above, the Audit Committee is governed by the Audit Committee Charter. A copy of this Charter is available on our website at www.uniqure.com under “Investors & Newsroom — Corporate Governance — uniQure Audit Committee Charter.” In addition to the risk oversight responsibilities discussed above, the Audit Committee’s other responsibilities include recommending the selection of our independent registered public accounting firm; reviewing with the Company’s independent registered public accounting firm the procedures for and results of their audits; reviewing with the independent accountants and management our financial reporting, internal controls and internal audit procedures; reviewing and approving related party transactions; and reviewing matters relating to the relationship between the Company and our independent registered public accounting firm, including the selection of and engagement fee for our independent registered public accounting firm, and assessing the independence of the independent registered public accounting firm. The Audit Committee has the authority to engage independent legal, accounting and other advisers, as it determines necessary to carry out its duties.
The Audit Committee met 65 times during 2016.2019.
Compensation Committee
The Compensation Committee is currently comprised of Madhavan Balachandran, Jack Kaye whoand David Meek.
Mr. Balachandran serves as chair and Paula Soteropoulos. Sander van Deventer served as a memberthe Chair of the committee and was chair prior to his appointment as chief scientific officer in August 2017. In the event of his successful election at the Extraordinary Meeting, the Board anticipates that Mr. Balachandran will be appointed to the Compensation Committee. Each of Mr. Balachandran, Mr. Kaye, Ms. Soteropoulos and Mr. BalachandranMeek satisfies the director independence standards and the independence standards for members of the Compensation Committee established by the SEC and Nasdaq. The Compensation Committee is governed by the Compensation Committee Charter. A copy of this Charter is available on our website at www.uniqure.com under “Investors & Newsroom — Corporate Governance — uniQure Compensation Committee Charter.” In addition to the risk oversight responsibilities discussed above, the Compensation Committee’s other responsibilities include reviewing and approving or recommending to the Board for approval, as appropriate, the compensation of our executive officers following consideration of corporate goals and objectives relevant to such executive officers; overseeing the evaluation of the Company’s senior executives; reviewing and making recommendations to the Board regarding incentive compensation and equity-based plans; and administering our stock option plans.
Without further action from the Board, the Compensation Committee has the authority to retain compensation consultants and other outside advisors to assist in the evaluation of executive officer compensation and is empowered to pay compensation to such consultants and other outside advisors.
The Compensation Committee retained Willis Towers Watson to act as a compensation consultant during the year ended December 31, 2016. The compensation consultant provided assistance2019 to assist in designing and reviewing our management and director compensation programs. Willis Towers Watson’s engagement included reviewing base salaries, equity incentivesFor further information, please refer to “Compensation Discussion and other compensation for directors
and senior management, including against a peer group of companies. In making decisions regarding the form and amount of compensation to be paid to directors and senior management, the Compensation Committee considered the information gathered by and recommendations of Willis Towers Watson. The Compensation Committee has assessed the independence of Willis Towers Watson pursuant to SEC rules and Nasdaq listing rules and concluded that the work of Willis Towers Watson did not raise any conflicts of interest.Analysis,” below.
The Compensation Committee met 610 times during 2016.2019.
Nominating and Corporate Governance Committee
The Nominating and Corporate Governance Committee is currently comprised of Sander van DeventerJeremy Springhorn, Philip Astley-Sparke and Paula Soteropoulos. Dr. van DeventerSpringhorn currently serves as chairthe Chair of the Compensation Committee. In the event of his successful elections at the Extraordinary Meeting, the Board anticipates that Dr. Springhorn will be appointed to the Nominating and Corporate Governance Committee and will be appointed chairCommittee. Each of the committee. As of August 7, 2017, Dr. van Deventer joined the Company’s management team as its chief scientific officer. He has notified the Board that he will resign his position on the Board upon the election of one or both of the nominees at the Extraordinary Meeting. Each ofSpringhorn, Ms. Soteropoulos and Dr. Springhorn satisfiesMr. Astley-Sparke satisfy the independence
14
Table of Contents
standards established by SEC and Nasdaq. The Nominating and Corporate Governance Committee is governed by the Nominating and Corporate Governance Committee Charter. A copy of this Charter is available on our website at www.uniqure.com under “Investors & Newsroom—Newsroom — Corporate Governance — uniQure Nominating and Corporate Governance Committee Charter.” In addition to the risk oversight responsibilities discussed above, the Nominating and Corporate Governance Committee’s other responsibilities include identifying individuals qualified to become Board members and to recommend to the Board the nominees for director at annual general meetings of Shareholders;shareholders; recommending to the Board nominees for each Committee; developing and recommending to the Board corporate governance principles applicable to the Company; and leading the Board in its annual review of the Board’s performance.
The Nominating and Corporate Governance Committee met 37 times during 2016.2019.
Research and Development Committee
The Research and Development Committee is comprised of Leonard Post, Paula Soteropoulos, and Jeremy Springhorn. Dr. Post serves as the Chair of the Research and Development Committee. Each of Dr. Post, Ms. Soteropoulos and Dr. Springhorn satisfy the independence standards established by SEC and Nasdaq. The Research and Development Committee is governed by the Research and Development Committee Charter. A copy of this Charter is available on our website at www.uniqure.com under “Investors & Newsroom — Corporate Governance — uniQure Research and Development Committee Charter.” In addition to the risk oversight responsibilities discussed above, the Research and Development Committee’s other responsibilities include: serving as an advisory body to the Board in matters related to the Company’s technology, research and development activities, product pipeline, and manufacturing platform (the “Company’s Technology”); advising the Board on the strategic direction of the Company with respect to the Company’s Technology; and evaluating the function and effectiveness of the Company’s research, development, manufacturing operations, clinical operations, and other technical, scientific and medical operations.
The Research and Development Committee was formed in December 2019 and did not meet in 2019.
Polices Governing Director Nominations
Director Nomination Process
Our Board is responsible for selecting its own members.members for appointment. The Board delegates the selection and nomination process to the Nominating and Corporate Governance Committee, with the expectation that other members of the Board, and of management, will be requested to take part in the process as appropriate. The Nominating and Corporate Governance Committee makes recommendations to the Board regarding the size and composition of the Board. The Nominating and Corporate Governance Committee is responsible for ensuring that the composition of the Board accurately reflects the needs of the Company’s business and, in furtherance of this goal, for proposing the addition of members and the necessary resignation of members for purposes of obtaining the appropriate members and skills. The Nominating and Corporate Governance Committee recommends, and the Board nominates,non-executive directors nominate, candidates to stand for electionappointment as directors.
Generally, our Nominating and Corporate Governance Committee identifies candidates for director nominees in consultation with management, through the use of other advisors, through the recommendations submitted by shareholders or through such other methods as the Nominating and Corporate Governance Committee deems to be helpful to identify candidates. Candidates recommended by shareholders and other stakeholders are given appropriate consideration in the same manner as other candidates. Once candidates have been identified, our Nominating and Corporate Governance Committee confirms that the candidates meet all of the minimum qualifications for director nominees established by the Nominating and Corporate Governance Committee. The Nominating and Corporate Governance Committee may gather information about the candidates through interviews, detailed questionnaires, background checks or any other means that the Nominating and Corporate Governance Committee deems to be appropriate in the evaluation process. The Nominating and Corporate Governance Committee then meets as a group to discuss and evaluate the qualifications and skills of each candidate, both on an individual basis and taking into account the overall composition and needs of the Board. Based on the results of the
15
Table of Contents
evaluation process, the Nominating and Corporate Governance Committee recommends candidates as director nominees for electionappointment to the Board for the Board’s approval.
With respect to Dr. Springhorn and Mr. Balachandran were identified as candidates for positions on the Board by the members of the Nominating and Corporate Governance Committee, with the assistance of Mr. Astley-Sparke.
Qualifications
The Nominating and Corporate Governance Committee may receive from shareholders and others recommendations for nominees for electionappointment to the Board and recommend to the Board candidates for Board membership for consideration by the shareholders at the annual general meeting of shareholders. In recommending candidates to the Board, the Nominating and Corporate Governance Committee takes into
consideration the Board’s criteria for selecting new directors, including, but not limited to, integrity, past achievements, judgment, intelligence, relevant experience and a commitment to understanding the Company’s business and its industry and the ability of the candidate to devote adequate time to Board duties. The Nominating and Corporate Governance Committee does not assign specific weights to particular criteria, and no particular criterion is a prerequisite for any Board candidate. We do however consider diversity in reviewing director candidates and do not discriminate on the basis of race, religion, sexual orientation, sex or national origin. Under Dutch law, as a company with fewer than 30% of the directors being women, we are required to disclose the rationale behind our failure to have a specified diversity percentage for the Board and our efforts to obtain such diversity and have done so in our Dutch statutory annual report for 2016, which is available on our website. In order for the Board to fulfill its responsibilities, our Nominating and Corporate Governance Committee believes that the Board should include directors possessing a blend of experience, knowledge and ability, regardless of other characteristics.
Family Relationships
There are no family relationships among any of our directors,Any Shareholder wishing to recommend a candidate for Board membership should submit the recommendation in writing to Investor Relations at uniQure N.V., Paasheuvelweg 25a, 1105 BP Amsterdam, the Netherlands. The written submission should set forth the candidate’s qualifications as specified in the uniQure Nominating and Corporate Governance Committee Charter. The Nominating and Corporate Governance Committee will consider all candidates recommended by Shareholders who satisfy the minimum qualifications for director nominees for directors, or executive officers.
Compensation Committee Interlocks and Insider Participation
None of our executive officers currently serve, or have served during the last completed fiscal year, on the compensation committee or board of directors of any other entity that has one or more executive officers serving as aBoard member of our Board or Compensation Committee.attributes.
Code of Business Conduct and Ethics and Corporate Governance Guidelines and Board Rules
We have adopted a code of business conduct and ethics that is applicable to all of our employees, officers, and directors, including our chief executive officerChief Executive Officer and chief financial officer.Chief Financial Officer. The code of business conduct and ethics and corporate governance guidelines and board rules areis available on our website at www.uniqure.com . under “Investors & Newsroom — Corporate Governance —uniQure Code of Business Conduct and Ethics.” We have also adopted corporate governance guidelines and board rules which are applicable to the company’s management.management and are available on our website at www.uniqure.com under “Investors & Newsroom — Corporate Governance — uniQure Corporate Governance Guidelines and Rules for the Board of Directors”.
In addition to the Listing Rules of the NASDAQNasdaq Global Select Stock Market and rules and regulations as promulgated by the SEC, as a Dutch company, our governance practices are governed by the Dutch Corporate Governance Code. The Dutch Corporate Governance Code (as amended) contains a number of principles and best practices, with an emphasis on integrity, transparency, and accountability as the primary means of achieving good governance.
There is considerable overlap between the requirements we must meet under U.S. rules and regulations and the provisions of the Dutch Corporate Governance Code. Although we apply several provisions of the Dutch Corporate Governance Code, as a “domestic” issuer, we comply with the Nasdaq corporate governance requirements.
In accordance with the Dutch Corporate Governance Code’s compliance principle of “comply-or-explain,“apply-or-explain,” which permits Dutch companies to be fully compliant with the Dutch Corporate Governance Code by either applying the Dutch practices or explaining why the company has chosen to apply different practices, we disclose in our Dutch statutory annual report that accompanies our Dutch statutory annual accounts to what extent we do not apply provisions of the Dutch Corporate Governance Code, together with the reasons for those deviations. Our Dutch statutory annual report may be found on the “Investors & Newsroom — Events and Presentations” section of our website at http://www.uniqure.com/investors-newsroom/events-presentations.php.
SECTION 16(a) BENEFICIAL OWNERSHIP REPORTING COMPLIANCE
Section 16(a)16
Table of the Exchange Act requires our executive officers, directors and persons who beneficially own more than ten percent of our Ordinary Shares to file reports of their beneficial ownership and changes in ownership (Forms 3, 4 and 5, and any amendment thereto) with the SEC. Executive officers, directors, and greater-than-ten-percent holders are required to furnish us with copies of all Section 16(a) forms they file. We were a foreign private issuer until January 1, 2017 and therefore our executive officers, directors and persons who beneficially own more than ten percent of our Ordinary Shares were not required to file reports of their beneficial ownership or changes in beneficial ownership prior to January 1, 2017.Contents
Based solely upon a review of the Forms 3, 4, and 5, as applicable, furnished to us since we ceased to be a foreign private issuer on January 1, 2017, we have determined that our executive officers, directors, and greater-than-ten-percent beneficial owners filed their beneficial ownership and change in ownership reports with the SEC in a timely manner, except as listed below.
Reporting Person
|
| Filing Due Date
|
| Date Filed
|
| Filing
|
Philip Astley-Sparke
| | January 3, 2017
| | January 4, 2017
| | Form 3
|
Jonathan Garen
| | January 3, 2017
| | January 5, 2017
| | Form 3
|
Matthew Kapusta
| | January 3, 2017
| | January 5, 2017
| | Form 3
|
Maiken Keson-Brookes
| | January 3, 2017
| | January 5, 2017
| | Form 3
|
Maria E. Cantor
| | January 3, 2017
| | January 5, 2017
| | Form 3
|
Christian Meyer
| | January 3, 2017
| | January 6, 2017
| | Form 3
|
Harald Petry
| | January 3, 2017
| | January 6, 2017
| | Form 3
|
Paul Firuta
| | January 3, 2017
| | January 9, 2017
| | Form 3
|
Jack Kaye
| | January 3, 2017
| | January 10, 2017
| | Form 3
|
Alex Kuta
| | January 27, 2017
| | February 1, 2017
| | Form 3
|
Maria E. Cantor
| | January 31, 2017
| | February 21, 2017
| | Form 4/A
|
Paul Firuta
| | January 31, 2017
| | February 21, 2017
| | Form 4/A
|
Jonathan Garen
| | January 31, 2017
| | February 21, 2017
| | Form 4/A
|
Maiken Keson-Brookes
| | January 31, 2017
| | February 21, 2017
| | Form 4/A
|
Christian Meyer
| | January 31, 2017
| | February 21, 2017
| | Form 4/A
|
Harald Petry
| | January 31, 2017
| | February 21, 2017
| | Form 4/A
|
Matthew Kapusta
| | February 23, 2017
| | March 3, 2017
| | Form 4
|
CERTAIN RELATIONSHIPS AND RELATED PERSONS TRANSACTIONS
Pre-Approval Policy Regarding Related Party Transactions
The Board has adopted a related party transactions policy, pursuant to which the chief financial officerChief Financial Officer and the Audit Committee isare charged with reviewing and approving or disapproving related party transactions. A “Related Party Transaction” under the policy means any transaction, arrangement or relationship (or any series of similar transactions, arrangements or relationships) where the amount involved or proposed to be involved exceeds $120,000 (or its equivalent in any currency), in which the Company or any of its controlled subsidiaries was, is or will be a participant (i.e., not necessarily a party) and in which any Related Party, as defined below, had, has or will have a direct or indirect material interestinterest. The “Related Party Transactions Policy” supplements the provisions in the Company’s Code of Business Conduct and Ethics concerning potential conflict of interest situations. Pursuant to the policy, compensation of directors and senior management are reviewed and approved by the Compensation Committee.
This written policy covers transactions or series of transactions in which the Company or any subsidiary participates and a “Related Party” has or will have a direct or indirect material interest. For purposes of this policy, a “Related Party” is:
· Each director and executive officer of the Company and any person who was serving as a director and/or executive officer at any time since the beginning of the Company’s last fiscal year;
· Any nominee for electionappointment as a director of the Company;
· Any security holder who atis the timebeneficial owner or record holder of the occurrence of the transaction, owned beneficially or of record more than 5% of any class of the Company’s voting securities;
· Any immediate family member of any of the foregoing persons. An “immediate family member” includes the spouse, parents, stepparents, children, stepchildren, siblings, mothers- and fathers-in-law, sons- and daughters-in-law, brothers- and sisters-in-law, and any person (other than a tenant or employee) sharing the household of a director, executive officer, director nominee or greater than 5% security holder of the Company; and
· Any entity that employs any person identified in the above or in which any person identified in the above directly or indirectly owns or has a material interest.
Pursuant to the Related Party Transactions Policy, each Company executive officer, director or nominee for director or any other officer or employee who intends to cause the Company to enter into a related party transaction must fully disclose to the chief financial officerChief Financial Officer all material facts concerning a prospective transaction or arrangement involving the Company in which such person may have an interest. The chief financial officerChief Financial Officer will review the information and make a preliminary, written conclusion as to whether the transaction is a related party transaction. If the preliminary conclusion is that the transaction would be a related party transaction, the chief financial officerChief Financial Officer will present the information and his conclusion to the Audit Committee for review. If a member of the Audit Committee is involved in the transaction, that member will not participate in determining whether the related party transaction is approved or ratified by the Audit Committee. Annually, the Audit Committee will review any previously approved or ratified related party transactions that are continuing and determine based on then-existing facts and circumstances.
Before any related person transaction is approved, the following factors are to be considered:
· theThe Related Party’s interest in the transaction;
· theThe approximate value of the aggregate amount involved in the transaction;
· theThe approximate value of the amount of the Related Party’s interest in the transaction;
17
Table of Contents
· aA summary of the material terms of and facts relating to the transaction, including any documentation or proposed documentation for the transaction, and identification of the area(s) of the Company’s business directly relevant to the transaction;
· whereWhere the transaction involves the purchase or sale of products, property or services, the availability of comparable products, property or services from or to (as applicable) unrelated third-party sources;
· whetherWhether the transaction was undertaken in the ordinary course of business of the Company;
· anAn assessment of whether the transaction’s terms are comparable to terms available from or to (as applicable) unrelated third parties in an arms-length transaction;
· theThe purpose of, and the potential benefits to the Company of the transaction; and
· anyAny other information regarding the transaction or the Related Party in the context of the proposed transaction that would be material to investors in light of the circumstances of the particular transaction.
Approval of a transaction under the policy will be granted only if it is determined that, under all of the circumstances, the transaction is in, or not inconsistent with, the best interests of the Company.
Review of Related Party Transactions
Since January 1, 2016,2019, we have engaged in the following transactions with the members of our Board, senior management, parties that held more than 5% of our Ordinary Shares during that period, and their affiliates, which we refer to as our related parties. Each of these transactions was approved in accordance with our Related Transactions Policy.
Grants of Options to Related Parties
We grant options to members of the Board and senior management. Details of options granted are included within the beneficial ownership table below.
4D Molecular Therapeutics Collaboration
In January 2014, we entered into a collaboration and license agreement with 4D Molecular Therapeutics. 4D Molecular Therapeutics, or 4DMT. 4DMT is a company co-founded by Dr. David Schaffer, whoand he currently serves as the Chief Scientific Officer. Dr. Schaffer was appointed to our Board in January 2014 pursuant to the terms of that collaboration.collaboration and license agreement. In connection with this transaction, we agreed to provide specified research and development financing, areand were obligated to make certain upfront, royalty and milestone payments,payments. In August 2019, we entered into an amended and grantedrestated collaboration and license agreement (“Amended CLA”), as well as a separate new collaboration and license agreement (“New CLA”) with 4DMT. Pursuant to the terms of the Amended CLA, we received from 4DMT an optionexclusive sublicensable, worldwide license under certain 4DMT intellectual property rights to purchaseresearch, develop, make, use and commercialize previously selected AAV capsid variants and certain associated products using 4DMT proprietary AAV technology for delivery of gene therapy constructs to cells in the central nervous system and the liver (the “Field”). In accordance with the New CLA, the parties agreed to research and develop, at 4DMT’s cost, new AAV capsid variants using 4DMT proprietary AAV technology for delivery of up to 609,744 Ordinary Shares at an exercise pricesix additional transgene constructs in the Field that will be selected by us. Dr. Schaffer’s term of €0.05 per share. At October 1, 2014, 25%office as a non-executive director of the options vested (expiring at December 28, 2014), 50% of the options vested at January 31, 2015 (expiringCompany expired on December 28, 2015) and the remainder vested on January 31, 2016 (expiring on December 28, 2016).June 17, 2020.
BMS
In April 2015, we and Bristol Myers Squibb (“BMS”) entered into various commercial and investment agreements providing BMS exclusive access to uniQure’s gene therapy technology platform for multiple targets in cardiovascular and other target-specific disease areas. We received $50 million in upfront payments upon effectiveness of the licensing and collaboration transaction in May 2015. An additional $15 million payment was
18
Table of Contents
received in July 2015 upon designation of three additional collaboration targets by BMS. In addition, pursuant to the collaboration agreements, in June 2015, BMS purchased 1,112,319 of our Ordinary Shares for aggregate consideration of $37.6 million. Immediately after the issuance, BMS owned 4.9% of our outstanding Ordinary Shares. In August 2015, we issued an additional 1,275,789 of our Ordinary Shares to BMS for aggregate consideration of $37.9 million. Immediately after the issuance, BMS owned 9.9% of our outstanding Ordinary Shares. We recognized $3.9$5.0 million in license revenue from BMS for the year ended December 31, 2016 (2015: $2.42019 (2018: $7.5 million).
19
Table of Contents
SECURITY OWNERSHIP OF CERTAIN
BENEFICIAL OWNERS AND MANAGEMENT
Based on information publicly filed and provided to us by certain holders, the following table shows the number of our Ordinary Shares beneficially owned as of July 1, 2017,September 30, 2020 by (i) each person known by us to beneficially own more than five percent of our voting securities, (ii) each named executive officer, (iii) each of our directors, (iv) each of our director nominees, and (v) all of our current named executive officers and directors as a group. Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, Ordinary Shares that could be issued upon the exercise of outstanding equity awards and warrants held by that person that are currently exercisable or exercisable within 60 days of July 1, 2017 daysSeptember 30, 2020 are considered outstanding. As of July 1, 2017,September 30, 2020, we had 25,620,07344,487,510 Ordinary Shares outstanding. Unless otherwise stated in a footnote, each of the beneficial owners listed below has direct ownership of and sole voting power and investment power with respect to our Ordinary Shares.
Unless otherwise noted below, the address of each director and named executive officer is c/o uniQure N.V., Paasheuvelweg 25a, 1105BP1105 BP Amsterdam, the Netherlands.
Name and Address of | | Ordinary Shares Beneficially Owned | |
Beneficial Owner | | Number | | Percent | |
| | | | | |
5% or Greater Shareholders (“Major Shareholders”): | | | | | |
Entities affiliated with Forbion (1) | | 4,383,669 | | 17.10 | % |
| | | | | |
Bristol-Myers Squibb Company (2) | | 2,388,108 | | 9.32 | % |
| | | | | |
Coller International Partners V-A, L.P. (3) | | 5,373,768 | | 20.97 | % |
| | | | | |
Director Nominees | | | | | |
| | | | | |
Jeremy P. Springhorn, Ph.D. | | 0 | | 0 | % |
| | | | | |
Madhavan Balachandran | | 0 | | 0 | % |
| | | | | |
Directors and Named Executive Officers | | | | | |
| | | | | |
Matthew Kapusta | | 112,500 | | 0.40 | % |
| | | | | |
Philip Astley-Sparke | | 13,750 | | * | |
| | | | | |
Jack Kaye | | 2,500 | | * | |
| | | | | |
Will Lewis | | 17,500 | | * | |
| | | | | |
David Schaffer | | 7,534 | | * | |
| | | | | |
Paula Soteropoulos | | 20,000 | | * | |
| | | | | |
Sander van Deventer (1) | | 12,534 | | * | |
| | | | | |
Daniel Soland (4) | | 0 | | * | |
| | | | | |
Charles Richard (5) | | 0 | | * | |
| | | | | |
Deya Corzo (5) | | 0 | | * | |
| | | | | |
Equity awards of all directors and named executive officers as a group (11 persons) (6) | | 186,318 | | 0.73 | % |
| | | | | |
Directors and Named Executive Officers Total | | 284,692 | | 1.11 | % |
| | | | | |
Major Shareholders, Directors and Named Executive Officers Total | | 8,036,631 | | 31.36 | % |
Name and Address of | | Ordinary Shares Beneficially Owned | |
Beneficial Owner | | Number | | Percent | |
5% or Greater Shareholders (“Major Shareholders”): | | | | | |
ForUniQure B.V. (1) | | 4,386,741 | | 9.86 | % |
| | | | | |
FMR, LLC (2) | | 4,361,992 | | 9.81 | % |
| | | | | |
Federated Hermes Inc (3) | | 3,011,446 | | 6.77 | % |
| | | | | |
Redmile Group L.L.C (4) | | 2,553,325 | | 5.65 | % |
| | | | | |
Nantahala Capital Management, LLC (5) | | 2,486,838 | | 5.59 | % |
| | | | | |
Bristol-Myers Squibb Company (6) | | 2,388,108 | | 5.37 | % |
| | | | | |
Directors and Named Executive Officers | | | | | |
| | | | | |
Matthew Kapusta | | 685,253 | | 1.52 | % |
| | | | | |
Sander van Deventer, Ph.D. (8) | | 221,136 | | 0.49 | % |
| | | | | |
Alexander E. Kuta, Ph.D. | | 119,279 | | 0.27 | % |
| | | | | |
Philip Astley-Sparke | | 57,690 | | 0.13 | % |
| | | | | |
Jack Kaye | | 46,813 | | 0.11 | % |
| | | | | |
Paula Soteropoulos | | 38,108 | | 0.09 | % |
| | | | | |
Robert Gut, Ph.D. | | 39,817 | | 0.09 | % |
| | | | | |
Madhavan Balachandran | | 25,190 | | 0.06 | % |
| | | | | |
Jeremy P. Springhorn, Ph.D. | | 25,190 | | 0.06 | % |
| | | | | |
David Meek | | 16,136 | | 0.04 | % |
| | | | | |
Directors and Named Executive Officers Total (7)(8) | | 1,274,612 | | 2.79 | % |
| | | | | |
Major Shareholders, Directors and Named Executive Officers Total | | 20,463,062 | | 45.83 | % |
20
Table of Contents
* Denotes less than 1% beneficial ownership.
(1)The registered office of Forbion 1, ForUniQure and Forbion Management is Gooimeer 2-35, 1411DC Naarden, The Netherlands. The number of shares reported is based solely on the Schedules 13G/A filed by ForUniQure B.V. and Forbion I Management B.V. on February 14, 2017 and Forbion I Co II Management B.V. on February 15, 2017 and with respect to Coöperative AAC LS U.A., a review of the Company’s registered shareholders as of March 31, 2017.2020. Forbion’s beneficial ownership consists of (i) 987,6744,376,883 Ordinary Shares held by Coöperative AAC LS U.A.ForUniQure B.V., or Coöperative,ForUniQure and (ii) 1,530,5019,859 Ordinary Shares held by Forbion Co-Investment Coöperatief U.A., or FCI and (iii) 1,865,494 Ordinary Shares held by Forbion Co-Investment II Coöperatief U.A., or FCI II.Management. Forbion 1 Management B.V., or Forbion 1, the director of Coöperative and FCI,ForUniQure and Forbion 1 Co II Management B.V., the director of FCI II, and Forbion Capital Partners Management Services B.V., or Forbion Capital Partners, may be deemed to have voting and dispositive power over the Ordinary Shares held by Coöperative, FCIForUniQure and FCI II. Investment decisionsForbion Management. Forbion 1, the director of ForUniQure, has voting and investment power over the shares held by ForUniQure, which are exercised through Forbion’s investment committee, consisting of H. A. Slootweg, M. A. van Osch, G. J. Mulder and Dr. van Deventer. None of the members of the investment committee have individual voting and investment power with respect to such shares, and the Ordinary Shares held by Coöperative, FCI and FCI II can be made by any twomembers disclaim beneficial ownership of such shares except to the duly authorized representativesextent of Coöperative, FCI and FCI II.their proportionate pecuniary interests therein. In addition to serving on Forbion’s investment committee, Dr. van Deventer is a partner of Forbion Capital Partners, which acts as the investment advisor to the directors of Coöperative, FCIForUniQure and FCI II. Dr. van Deventer disclaims beneficial ownership of such Ordinary Shares, except to the extent of his pecuniary interest therein. The address of Forbion Capital Partners, Coöperative, FCI and FCI II is Gooimeer 2-35, 1411 DC Naarden, The Netherlands.1.
(2)The registered office of FMR, LLC is 245 Summer Street, Boston, Massachusetts 02210, United States. The number of shares reported is based solely on a Schedule 13G/A filed with the Securities and Exchange Commission by FMR, LLC on February 7, 2020.
(3) The registered office of Federated Hermes Inc. is 1001 Liberty Avenue, Pittsburgh, Pennsylvania 15222-3770, United States. The number of shares reported is based solely on a Schedule 13G/A filed with the Securities and Exchange Commission by Federated Hermes Inc on February 14, 2020.
(4) The registered office of Redmile Group LLC is One Letterman Drive Building D Suite D3-300, San Francisco, California 94129, United States. The number of shares reported is based solely on a Schedule 13G filed with the Securities and Exchange Commission by Redmile Group LLC on February 14, 2020.
(5) The registered office of Nantahala Capital Management, LLC is 130 Main St. 2nd Floor, New Canaan, Connecticut 06840, United States. The number of shares reported is based solely on a Schedule 13G/A filed with the Securities and Exchange Commission by Nantahala Capital Management, LLC on February 14, 2020.
(6) The registered office of Bristol-Myers Squibb Company Corp is 345 Park Avenue, New York, NYNew York 10154, United States. The number of shares reported is based solely on a Schedule 13G filed with the Securities and Exchange Commission by Bristol-Myers Squibb Company on August 17, 2015.
(3)(7) Coller International Partners V-A, L.P.’s beneficial ownership consists of (i) 990,099 Ordinary Shares held by Coller International Partners V-A, L.P., or Coller; (ii) 987,674 Ordinary Shares held by Coöperative; (iii) 1,530,501 Ordinary Shares held by FCI; and (iv) 1,865,494 Ordinary Shares held by FCI II. Coller is a limited partner of the Forbion funds. Coller has no dispositive or voting power over ordinary shares held by the Forbion funds and disclaims beneficial ownership of such ordinary shares except to the extent of its pecuniary interest therein. See footnote 1 above. The general partner of Coller is Coller International General Partner V, L.P. of which Coller Investment Management Limited, or CIML, is the general partner. The directors of CIML are Jeremy Joseph Coller, Cyril Joseph Mahon, Roger Alan Le Tissier, Paul McDonald, Peter Michael Hutton, John Charlton Loveless and Andrew Thane Maden Hitchon and may be deemed to share voting and dispositive power with respect to the ordinary shares held by Coller. The CIML directors disclaim beneficial ownership of such ordinary shares except to the extent of their pecuniary interest therein. The address of Coller is c/o Coller Investment Management Limited, PO Box 255, Trafalgar Court, Les Banques, St Peter Port, Guernsey, Channel Islands.
(4) Mr Soland served as our chief executive officer from December 2015 until September 2016.
(5) Dr. Richard’s and Dr. Corzo’s employments ended effective December 31, 2016.
(6)Includes for the persons listed below the following ordinary sharesOrdinary Shares subject to options held by that person that are currently exercisable or become exercisable within 60 days of July 1, 2017 and ordinary shares issuable upon the vesting of RSU awards within 60 days of July 1, 2017:September 30, 2020 as well as Ordinary Shares:
Name | | Options | | RSU Awards | | | Options | | Ordinary shares | |
Matthew Kapusta | | 112,500 | | 0 | | | 517,271 | | 167,982 | |
Sander van Deventer, Ph. D. | | | 210,113 | | 11,023 | |
Alexander E. Kuta, Ph.D. | | | 113,797 | | 5,482 | |
Philip Astley-Sparke | | 8,750 | | 5,000 | | | 51,685 | | 6,005 | |
Jack Kaye | | 2,500 | | 0 | | | 32,685 | | 14,128 | |
Will Lewis | | 12,500 | | 5,000 | | |
David Schaffer | | 5,000 | | 2,534 | | |
Paula Soteropoulos | | 15,000 | | 5,000 | | | 35,685 | | 2,423 | |
Sander van Deventer (1) | | 10,000 | | 2,534 | | |
Daniel Soland (3) | | 0 | | 0 | | |
Charles Richard (4) | | 0 | | 0 | | |
Deya Corzo (4) | | 0 | | 0 | | |
Robert Gut, Ph.D. | | | 32,502 | | 7,320 | |
Madhavan Balachandran | | | 19,185 | | 6,005 | |
Jeremy Springhorn, Ph.D. | | | 19,185 | | 6,005 | |
David Meek | | | 13,638 | | 2,508 | |
Directors and Named Executive Officers Total | | 166,250 | | 20,068 | | | 1,045,736 | | 238,608 | |
(8) Dr. van Deventer left the Company on September 14, 2020.
21
Table of Contents
Securities Authorized for Issuance under Equity Compensation Plans
The table below provides information about our Ordinary Shares that may be issued under our 2014 Amended and Restated Share Option Plan (the “2014 Restated Plan”), our predecessor plans and outside these plans as of July 1, 2017:September 30, 2020:
Plan Category | | (a)Number of securities to be
issued upon exercise of
outstanding options,
warrants and rights | | (b) Weighted-average
exercise price of outstanding
options, warrants and rights (1) | | (c) Number of securities
remaining available for
future issuance under equity
compensation plans
(excluding securities reflected
in column (a)) | | | (a) Number of securities
to be
issued upon exercise of
outstanding options,
warrants and rights | | (b) Weighted-average
exercise price of outstanding
options, warrants and rights (1) | | (c) Number of securities
remaining available for
future issuance under
equity
compensation plans
(excluding securities reflected
in column (a)) | |
2012 Equity Incentive Plan (Equity Compensation Plan Approved by Security Holders) | | 196,702 | | $ | 9.28 | | 0 | | | 14,000 | | $ | 9.49 | (2) | — | |
2014 Restated Plan (Equity Compensation Plan Approved by Security Holders) | | 2,766,289 | | $ | 5.06 | | 1,575,764 | | | 2,848,158 | | $ | 22.15 | (3) | 2,230,615 | |
Equity Compensation Plans Not Approved by Security Holders (2)(4) | | 234,750 | | $ | 8.26 | | — | (3) | | 98,000 | | $ | 5.31 | | — | (5) |
Total | | 3,197,741 | | $ | 5.56 | | 1,575,764 | | | 3,632,963 | | $ | 21.65 | | 2,230,615 | |
(1) The exercise price for our RSU and PSU awards is $0.00 and is included in the weighted-average exercise price of outstanding options, warrants and rights.
(2) The exercise price of outstanding options is denominated in euro and translated to dollars at the foreign exchange rate as of September 30, 2020.
(3) These PSU Awards are measured at target for the outstanding performance-based awards.
(4)These awards include inducement grants entered into by the Company outside of the 2014 Restated Plan and the predecessor plans.
(3)(5) At the 2016 annual general meeting of shareholders held on June 15, 2016,2020 Annual General Meeting, our Board was granted the authority to issue a maximum of 19.9% of the Company’s aggregate issued capital outside of a public offering. As of July 1, 2017, this consisted of 5,100,385 Ordinary Shares. These Ordinary Shares may be issued as part of inducement or other option grants but are not restricted to that purpose.
22
Table of Contents
MANAGEMENT COMPENSATION COMMITTEE REPORT
The Compensation Committee Report is not “soliciting material,” is not deemed “filed” with the SEC and is not to be incorporated by reference into any filing of the Company under the Securities Act of 1933 or the Securities Exchange Act of 1934, both as amended.
We have reviewed and discussed the Compensation Discussion & Analysis contained in this Proxy Statement with uniQure’s management, and based upon such review and discussion, we recommended to the Board that the Compensation Discussion & Analysis be included in this Proxy Statement.
The Compensation Committee
/s/ Madhavan Balachandran | |
Madhavan Balachandran | |
| |
/s/ Jack Kaye | |
Jack Kaye | |
| |
/s/ David Meek | |
David Meek | |
23
Table of Contents
COMPENSATION DISCUSSION & ANALYSIS
This section discusses the principlesCompensation Discussion and Analysis (the “CD&A”) explains our compensation philosophy, policies underlying our executive compensation programand decisions for our named executive officers. The Compensation Committee oversees our executive compensation programs and approves or makes recommendations to the Board2019 for approval where appropriate and required by the Compensation Committee’s charter. In this role, the Compensation Committee reviews and approves all compensation decisions relating to our named executive officers. For fiscal 2016, the following wereexecutives, whom we refer to in this CD&A and in the following tables as our named executive officers:
NameNamed Executive Officer
| | PositionTitle
|
Matthew Kapusta | | Chief Executive Officer and interim Chief Financial Officer (1) |
Daniel SolandDr. Robert Gut
| | Chief ExecutiveMedical Officer (1)(until October 14, 2020) |
Charles RichardDr. Alexander Kuta
| | SVP, Research and Development Neuroscience (2)Executive Vice President, Operations
|
Deya CorzoDr. Sander van Deventer
| | SVP, Therapeutic area head Liver/Metabolism (3)Executive Vice President, Product and Research Development (until September 14, 2020)
|
(1) Mr Soland servedExecutive Summary Our Business
We are a leader in the field of gene therapy, seeking to develop one-time administered treatments with potentially curative results for patients suffering from genetic and other devastating diseases. We are working to advance a focused pipeline of innovative gene therapies that have been developed both internally and through partnerships, such as chief executive officer fromour collaboration with Bristol Myers-Squibb focused on cardiovascular diseases. We believe our gene therapy technology platform and manufacturing capabilities provide us distinct competitive advantages, including the beginningpotential to reduce development risk, cost and time to market. We produce our adeno- associated virus based, or AAV-based, gene therapies in our own facilities with a proprietary, commercial-scale, current good manufacturing practices (“cGMP”) and compliant, manufacturing process. We believe our Lexington, Massachusetts-based facility is one of the year until September 2016. Mr. Kapusta was appointed as chief executive officer on an interim basis from September 2016 and permanently from December 2016.
(2) Dr. Richard’s employment ended effective December 31, 2016.
(3) Dr. Corzo’s employment ended effective December 31, 2016.world’s leading, most versatile, gene therapy manufacturing facilities.
Objectives2019 Performance and Achievements
In 2019 and early 2020, our named executive officers played critical roles in the achievement of our goal to advance and expand our pipeline of leading gene therapy product candidates.
We had significant achievements related to our lead product candidate, etranacogene dezaparvovec or AMT-061. In January 2019, the first patient was treated in our HOPE-B pivotal study of etranacogene dezaparvovec in hemophilia B, after completing the six-month lead-in phase. In September 2019, we achieved full patient enrollment in the lead-in phase of our HOPE-B study. Additionally, following the completion of the dosing of a Phase IIb dose-confirmation study of etranacogene dezaparvovec in 2018, we were able to announce updated data on several occasions throughout the year. The 52-week follow-up data in the Phase IIb study showed that all three patients had stabilized and sustained factor IX activity at therapeutic levels after the one-time administration of etranacogene dezaparvovec.
We also achieved significant milestones in our AMT-130 program for our recombinant AAV5 vector carrying a DNA cassette encoding a microRNA that non-selectively lowers or knocks-down human huntingtin protein in Huntington’s disease patients. In January 2019, we announced that the U.S. Food and Drug Administration (FDA) completed its review of the Company’s Executive Investigational New Drug (IND) application for AMT-130. Following that review, our IND became effective, which allowed us to begin our planned Phase I/II study. In April 2019, the U.S. Food and Drug Administration (FDA) granted Fast Track designation for AMT-130. Preparations are underway to initiate the world’s first clinical study of a one-time administered therapy for the treatment of Huntington’s disease.
Additionally, in September 2019, we also took a significant step in providing long-term financial stability for the Company by closing on our underwritten public offering of a total of 5,625,000 of our ordinary shares at a public offering price of $46.00 per share. The gross proceeds to the Company from the offering, before deducting the underwriting discounts and commissions and estimated offering expenses payable by uniQure, was approximately $259 million, which we expect will provide the Company with funding into the 2022 fiscal year.
24
Table of Contents
Compensation ProgramsPhilosophy and Principles
As determined byWe operate in a competitive, rapidly changing and heavily-regulated industry. The long-term success of our business requires us to be resourceful, adaptable, and innovative. The skills, talent, and dedication of our executive officers are critical components to our success and the Compensation Committee,future growth of the Company’scompany. Therefore, our compensation programsprogram for its senior managementour executive officers, including our named executive officers, is designed to achieveattract, retain, and incentivize the following objectives:best possible talent.
The Compensation Committee has established core objectives for our compensation programs, which are underpinned by a focus on elements that attract and retain the talent we believe is necessary to successfully lead uniQure and our employees globally
·Pay for performance motivate
Motivate and reward our senior management to achieve established business and individual objectives
Align interests with our shareholders
Align compensation with the Company’s annualvalue realized by our shareholders
Use “at risk” compensation to incentivize executives
Use “at risk,” or variable, compensation to align the interests with those of our shareholders over time and contribute to the achievement of both short- and long-term corporate objectivesgoals
Attract and strategies;retain talented executives
· provide
Provide compensation opportunities and policies that are competitive with similarly sized biotechnology companies;
· align executive interests with those of our Shareholders; and
· attract and retain talented executives.companies
Elements ofHow We Determine Executive Compensation Compensation Oversight
At the 2016 Annual General Meeting, the annual meeting of Shareholders adopted a remuneration policy (the “Remuneration Policy”), which structures the compensation granted to our senior managers. The Company’s current executive compensation package for senior management focuses on a fixed base salary, short-term incentives (cash bonus), long-term incentives (equity awards), pension benefits and other benefits. Senior managers are also eligible to receive severance payments under certain circumstances, as further described below. We utilize base salary to incentivize company and individual performance in relation to competitive market conditions. Short-term incentives are tied to the achievement of pre-determined performance criteria. Our long-term incentives consist of annual and periodic equity awards linked to continued employment and, at the Board’s discretion, the achievement of certain performance targets. Severance and change in control benefits are used to help ensure we retain our executive talent.
The Compensation Committee determines, in its sole discretion,is composed solely of independent directors, who at the appropriate componentsend of senior management’s compensation package. As described2019 were Madhavan Balachandran, Jack Kaye and David Meek, with Mr. Balachandran serving as the Committee Chair. The Chair of the Board, Philip Astley-Sparke is invited to attend meetings, but is not a formal member.
Details of the Compensation Committee’s duties are fully set out in the sectionCompensation Committee’s charter, which can be found on our website: http://uniqure.com/investors-newsroom/corporate-governance.php.
The overarching purpose of the Proxy titled “Compensation Committee”, the Compensation Committee retained Willis Towers Watsonis to act asoversee the manner in which the Board discharges its responsibilities relating to uniQure’s compensation consultant duringpolicies, plans and programs for uniQure’s executive officers and directors.
The Compensation Committee is wholly accountable for any changes in compensation for the year ended December 31, 2016. Our senior managers’ overallChief Executive Officer, and the Chief Executive Officer is not included in any discussions regarding changes to his own compensation. For other named executive officers, recommendations are made by the Chief Executive Officer and subsequently reviewed and approved by the Compensation Committee. Overall compensation for our named executive officers may increase or decrease year-to-year based upon, among other things, his or her annual performance or changes in his or her responsibilities.
The Annual Committee Process
The Compensation Committee typically meets six or more times a year to consider the following items:
Quarter |
| Typical Meeting Topics |
|
Q1 | | · Determine corporate goals for current year; | |
25
Table of Contents
| | · Determine executive compensation for current year, including base salary, and bonus for prior year, target bonus for current year, and long-term equity incentives; · Determine director compensation, including cash and equity compensation; and · Determine employee equity grants; and adopt terms of annual incentive bonus plan for current year. | |
| | | |
Q2 | | · Assess prior year activities and Compensation Committee performance; and · Plan compensation cycle through remainder of current year and into following year. | |
| | | |
Q3 | | · Review Compensation Committee Charter; · Review with compensation consultant best practices related to disclosure and director and executive compensation; and · Engage compensation consultant for work associated with upcoming compensation cycle. | |
| | | |
Q4 | | · Review compensation peer group; · Review information provided by compensation consultant, including comparable data related to director and executive compensation; and · Perform initial evaluations for the year-ahead of target executive compensation (including cash and equity compensation), director compensation (including cash and equity compensation), employee equity grants, and terms of annual incentive bonus plan for upcoming year. | |
Additional meetings are scheduled on an as needed basis, and in 2019 the Committee met 10 times.
Use of an Independent Advisor
As set out in its Charter, the Compensation Committee has the authority to retain outside consultants to provide independent advice to the Committee. In 2019 the Committee retained Willis Towers Watson (“WTW”) as its independent compensation consultant. WTW reported directly to the Compensation Committee and took direction from the Chair of the Committee. Having assessed WTW’s independence pursuant to SEC rules and Nasdaq listing rules, the Compensation Committee concluded that the work of WTW did not raise any conflicts of interest.
During the year, WTW provided assistance in designing and reviewing our management and director compensation programs, including reviewing the compensation peer group, providing market data on all aspects of compensation, reviewing long-term incentive grant practices, and attended Compensation Committee meetings and provided general advice.
The Compensation Committee considered the analysis and advice from WTW, as well as support and insight from management when making compensation decisions.
Managing Compensation-Related Risk
uniQure operates in a highly regulated and competitive sector, and managing risk is embedded in the manner in which the Company is run and operates. The Board has delegated to the Compensation Committee responsibility to oversee compensation-related risk.
The Compensation Committee annually evaluates whether there are potential risks arising from the Company’s compensation policies and practices as part of our annual risk assessment performed by management and reported to and discussed with the Board. The Compensation Committee has determined that uniQure’s compensation policies and practices do not encourage executives to take excessive risks given that the various elements of the policies and practices diversify the risks associated with any single element of the executives’ compensation.
Compensation Peer Group
Given the fast-paced nature of our sector, the Compensation Committee reviews the constituents of the compensation peer group on an annual basis, with the support of WTW, to ensure they remain relevant and
26
Table of Contents
appropriate for comparisons. The Compensation Committee, with advice received from WTW, selected companies for our 2019 peer group through a screening process that considered publicly traded biopharmaceutical companies similar to us in number of employees, market capitalization and stage of product development. Based on the screening criteria, nine companies were removed from the peer group compared to the prior year, and eight new companies were added.
Retained Companies (n=9) |
| Removed Companies (n=9) |
| New Companies (n=8) |
· Arrowhead Pharmaceuticals | | · Adverum Biotechnologies | | · Alder Biopharmaceuticals |
· Blueprint Medicines | | · Applied Genetic Technologies | | · Arena Pharmaceuticals |
· Dynavax Technologies | | · Celldex Therapeutics | | · Denali Therapeutics |
· Epizyme | | · Concert Pharmaceuticals | | · Editas Medicine |
· Invitae | | · Genocea Biosciences | | · Intellia Therapeutics |
· Regenxbio | | · NewLink Genetics | | · MyoKardia |
· Revance Therapeutics | | · Vital Therapies | | · Voyager Therapeutics |
· Sangamo Therapeutics | | · T2 Biosystems | | · Wave Life Sciences |
· Spark Therapeutics | | · XBiotech | | |
The primary reasons for an exclusion or addition was to reflect therapeutic relevance or to meet appropriate size parameters. As a result, the 2019 compensation peer group, which was approved in September 2018, comprised the following 17 companies:
· Alder Biopharmaceuticals | | · Editas Medicine | | · Sangamo Therapeutics |
· Arena Pharmaceuticals | | · Epizyme | | · Spark Therapeutics |
· Arrowhead Pharmaceuticals | | · Intellia Therapeutics | | · Voyager Therapeutics |
· Blueprint Medicines | | · Invitae | | · Wave Life Sciences |
· Denali Therapeutics | | · MyoKardia | | |
· Dynavax Technologies | | · Regenxbio | | |
| | · Revance Therapeutics | | |
The peer companies had market capitalizations that ranged from approximately $697 million to $3.03 billion and a number of employees that ranged from 89 to 594. At the time the analysis was conducted, we had approximately 202 employees and a market capitalization of approximately $1.46 billion.
The Compensation Committee determined that uniQure’s size relative to the peer group was appropriate for the purpose of compensation comparisons. At the time of approval, uniQure ranked at the 57th percentile for market capitalization, at the 99th percentile for one-year Total Shareholder Return (“TSR”), at the 54th percentile for revenue and at the 88th percentile for headcount. For roles where insufficient proxy statement data was available to inform market comparisons, the Committee additionally referenced survey data provided by WTW and Radford for similarly sized biotech and biopharma companies.
Compensation Elements
At the 2016 Annual General Meeting, uniQure shareholders approved our Remuneration Policy, which sets out the structure for the compensation granted to our senior managers, including the Chief Executive Officer and other named executive officers. The full policy can be found on our website: http://uniqure.com/investors-newsroom/corporate-governance.php.
In summary, our compensation program is designed to be straightforward in nature with five core elements, the first three of which are compensation related and the last two are benefits reflecting local market practices for each named executive officer.
27
Table of Contents
Element |
| Purpose |
| Key Features |
Base Salary | | Provide market-competitive fixed compensation
Attract exceptional talent in the relevant market | | · Fixed cash compensation · Reviewed annually · Value informed by market levels for executives with comparable qualifications, experience and responsibility, coupled with the nature, scope and impact of the role · Target approximately 50th percentile of market peers, considering the factors noted above |
| | | | |
Short-Term Incentive
(Annual Cash Bonus) | | Reward for achievement of pre-defined criteria in areas of strategic importance to uniQure
Align compensation with Company performance | | · Subject to the approval of the Board in its discretion · Discretionary variable cash compensation ranging from 35% to 55% of annual Base Salary in 2019 · Maximum opportunity capped at 150% of target · Objectives based solely on corporate performance for the Chief Executive Officer, and a combination of corporate (80%) and individual (20%) performance for the other named executive officers · Corporate and individual targets established in the beginning of each year · Assessment against the predetermined targets informs actual cash bonus that is awarded · Target opportunity informed by levels in the market, with reference to the 50th — 75th percentile |
| | | | |
Long-Term Incentives
(Equity Awards) | | Align long-term interests with shareholders
Reward sustainable value creation
Encourage retention | | · Subject to the approval of the Board in its discretion · Annual awards of variable equity-based compensation · 2019 awards were a mix of stock options, restricted stock units and performance stock units · Stock options have a ten-year term, with 25% vesting after one year and then rateably on a quarterly basis · Restricted stock units vest rateably on an annual basis over three years · Performance stock units are earned based on the Company’s performance against corporate objectives, as determined and assessed by the Board. These awards have a pay-out range of 0% - 150% of target and vest after three years |
| | | | |
Pension and Retirement Savings Plans | | Provide market-competitive retirement benefits | | · Based on local market practice · U.S.-based named executive officers are eligible to participate in a qualified 401(k) Plan with matching of up to 3% of base salary · Netherlands-based named executive officers are eligible to participate in a defined contribution pension plan |
| | | | |
Other Benefits | | Provide market competitive benefits focused on well-being | | · An Employee Stock Purchase Plan (“ESPP”) is offered to all eligible employees, which includes eligible named executive officers · ESPP allows for purchase of discounted Ordinary Shares through accumulated payroll deductions |
28
Table of Contents
| | | | · Medical, dental and vision health care plans with premiums paid by the company for U.S.-based named executive officers · Up to four weeks of paid time off · Company-paid life insurance and short-term and long-term disability, with some employee contribution · Tuition reimbursement · Fitness membership reimbursement |
Target Pay Mix
A significant portion of our named executive officers’ target compensation is variable and at-risk, short term incentives (“STI”) and long term incentives (“LTI”) maximizing alignment with our shareholders and long-term value creation.
In 2019, the target compensation mix for the CEO, of which 85% was at-risk, is detailed below:
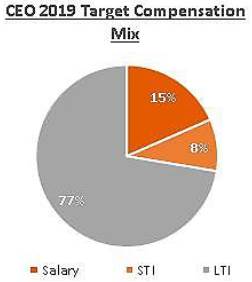
We do not specify a target mix of salary, STI and LTI compensation for our other named executive officers, but we use target a range of approximately 60% - 65% for the at-risk components. The overall compensation structure is adjusted to determine an appropriate mix on a position-by-position basis based on peer group data and other comparable compensation data for each position.
2019 Compensation Decisions and Outcomes Base Salary
As described below, our senior managersnamed executive officers receive a base salary, the terms of which are subject to each of their individual employment agreements. Adjustments to base salary may be based upon a number of factors, pursuant to the Company’s standard practices, including seniority, scope of responsibilities, individual performance, contributions to the Company and the Company’s overall financial and stock price performance. The Compensation Committee annually reviews each senior manager’snamed executive officer’s base salary and may adjust such senior manager’sindividual’s base salary after considering his or her responsibilities, performance and contributions to the Company and the Company’s overall performance. Additionally, the Compensation Committee will consider market data, with a view to ensuring base salary is set competitively, with a philosophy of targeting approximately the 50th percentile, taking into consideration the above factors. Based on that analysis and the recommendation of our Compensation Committee, the Board made adjustments from the prior year to the base salaries of our executive officers.
The 2019 base salary for our named executive officers are described below:
29
Short-Term Incentives (Annual Cash Bonus)Table of Contents
Named Executive Officer | | Base Salary | | Effective Date | |
Matthew Kapusta | | $ | 550,000 | | January 2019 | |
Robert Gut | | $ | 429,646 | | January 2019 | |
Alexander E. Kuta | | $ | 429,646 | | August 2019 | |
| | $ | 397,838 | | January 2019 | |
Sander van Deventer | | € | 348,000 | | August 2019 | |
| | € | 202,400 | | January 2019 | |
As part of our organizational realignment associated with the departure at that time of Dr. Scott McMillan, our Chief Operations Officer, Dr. Kuta and Dr. van Deventer were promoted during the year. Dr. Kuta’s base salary was increased in August 2019 in association with his promotion from the role of Senior Vice President, Regulatory to Executive Vice President, Operations. Dr. van Deventer’s base salary was increased in August 2019 in association with his promotion from the role of Chief Scientific Officer to Executive Vice President, Product and Research Development. Dr. van Deventer’s base salary as of January 2019 was based on a part-time schedule of 60% of full-time employment and as of September 2019 was based on a part-time schedule of 80% of full-time employment.
Short-term Incentive Bonus
The Company’s short-term incentives to senior managers consist of discretionary annual cash bonuses. The annualnamed executive officers provide an opportunity for our named executive officers to earn a cash bonus, for senior managers is linked tocontingent on the successful achievement of pre-determined performance targets approved by the Board.goals with various program areas aligned to our strategic objectives. The award of any bonuses shall be subject to the approval of the Board in its discretion.
Any bonus for the Chief Executive Officer is based solely on the assessment of company-wide performance. For the other named executive officers 80% of their opportunity is based on the same company-wide performance, with the remaining 20% based on individual performance.
Bonus opportunities for the named executive officers in 2019 were as follows:
Named Executive Officer | | Target Bonus (% of salary) | | Maximum Bonus (% of salary) | |
Matthew Kapusta | | 55 | % | 82.5 | % |
Robert Gut | | 40 | % | 60.0 | % |
Alexander E. Kuta | | 40 | % | 60.0 | % |
Sander van Deventer | | 40 | % | 60.0 | % |
Effective January 1, 2019 we increased the target bonus rate of Matthew Kapusta from 50% to 55%. We also increased the target bonus rate of Dr. Kuta from 35% to 40% upon his promotion to Executive Vice President, Regulatory. The target bonus rates of Dr. Gut and Dr. van Deventer remained unchanged at 40% during 2019.
Annually, we evaluate and establish performance targets based on the corporate goals that are adopted by the Board. Our performance targets are generally based on the achievement of a key set of core objectives considered essential to our successful performance over a given calendar year. These core objectives are designed across the range of functions of the Company, including clinical, research and technology, regulatory, manufacturing, finance and other general and administrative functions. Our performance against targets are reviewed periodically with the Board throughout the year. At the end of the calendar year, we assess the overall performance, which is then used for compensation decisions, including the payment of annual incentive bonuses.
In 2019, the Board approved the following corporate objectives.
Key Goal |
| Weighting |
| Why it Matters |
Advance our hemophilia B Program | | 50% | | Our AMT-061 product candidate for the treatment of hemophilia B is our lead product candidate. |
| | | | |
Advance our Huntington’s Disease Program | | 30% | | Our AMT-130 product candidate for the treatment of Huntington’s disease is entering the clinical phase. |
30
Table of Contents
Key Goal |
| Weighting |
| Why it Matters |
Advance our hemophilia A Program | | 10% | | Our AMT-180 product candidate for the treatment of Hemophilia A is in the late stages of pre-clinical development. |
| | | | |
Advance our Research and Technology Programs and Corporate Development | | 10% | | The development of enabling technologies and additional product candidates is core to our strategy. Enabling technologies include novel gene therapy components, such as AAV vectors and promoters, administration techniques and manufacturing capabilities. Our research pipeline is currently focused on liver-directed and CNS disorders, including gene therapies targeting hemophilia A, Fabry disease and spinocerebellar ataxia Type 3. |
| | | | To facilitate our goals, it is also critical that we manage our corporate resources effectively, as well as develop an infrastructure and organization that anticipates emergent needs. |
We believe these four strategic areas are critical to the successful execution of our long-term strategy and the achievement of sustainable shareholder value creation. In approving the targets, each goal within a program area has an associated level of achievement and time frame. The extent to which the goal is achieved, and whether or not it is on time, informs the rating assigned at year-end.
In order to achieve a threshold bonus the total performance must be assessed at a minimum of 50%. Amounts between threshold, target and maximum payout are interpolated to reward incremental achievement and no amounts are paid for results on a particular performance metric if actual results are below threshold. For performance assessed at below 50%, no bonus is paid, and for performance assessed at above 150%, no additional bonus is paid.
For the 2019 annual incentive bonus plan, our Board determined, based on the recommendation of the Compensation Committee, that the overall achievement of the Company relative to the target performance objectives was 109%. A summary of the performance assessment is below:
Key Goal |
| Assessment |
Advance our hemophilia B Program | | The Board determined that there was overachievement based on: · successful dosing of the first patient in our HOPE-B Phase III pivotal study of etranacogene dezaparvovec; · exceeding expectations for patient dosing in the HOPE-B study during 2019; · achieving full patient enrollment in the lead-in phase of the HOPE-B study; · activation of all clinical sites in the HOPE-B study; · completion of a Board-approved commercial plan; · receipt of FDA orphan drug designation; · validation of an in vitro diagnostic; and · acceptance of the data from our Phase IIb trial for publication in a prestigious journal. The overall contribution to the final assessment was 62%. |
| | |
Advance our Huntington’s Disease Program | | The Board determined there was partial achievement of our corporate goals based on: · achieving U.S. Food and Drug Administration (“FDA”) clearance of our Investigational New Drug Application for AMT-130; · receiving fast track designation from the FDA; · initiating patient screening in the Phase I/II study; |
31
Table of Contents
| | · executing on a defined scientific communications plan; and · releasing clinical product for use in the Phase I/II study. The overall contribution to the final assessment was 28%. |
| | |
Advance our hemophilia A Program | | The Board determined there was partial achievement of our corporate goals based on: · finalization of our phase 1/2 clinical protocol; and · executing on a defined scientific communications plan. The overall contribution to the final assessment was 2%. |
| | |
Advance our Research and Technology Programs | | The Board determined there was partial achievement of our corporate goals based on: · completing a proof-of-concept study in a diseased animal model of Fabry disease; · completing the characterization of a potential new liver-directed gene therapy program; and · developing specified new manufacturing capabilities and technologies, including proof of concept for a producer-cell line. The overall contribution to the final assessment was 3.5%. |
| | |
Advance Corporate Development Initiatives | | The Board determined there was overachievement of our corporate goals based on:
completing our follow-on public offering of $259 million; · achieving financial results within budget; and; · other items associated with the internal operations of the Company.
The overall contribution to the final assessment was 6.0%.
|
The remaining component of the target performance objectives was a discretionary assessment by the Board adding an additional 7.5% to the corporate performance for 2019. This was based on a number of additional factors not captured in the corporate goals, including successfully completing a facility expansion in Lexington Massachusetts, successfully manufacturing at 500L in accordance with current Good Manufacturing Practices, initiating and advancing new research programs focused on CNS disorders, the prosecution and issuance of new intellectual property for key programs, completing an organizational realignment involving the operations function and successfully transitioning to our new auditors at KPMG.
In respect of the individual performance component for the named executive officers other than the Chief Executive Officer, the Compensation Committee noted the following achievements in approving the rating recommendation submitted by the Chief Executive Officer:
Named Executive Officer |
| Individual Goal Assessment |
| Primary Achievements |
Matthew Kapusta | | Not applicable | | Not applicable |
| | | | |
Robert Gut | | Exceeded goals | | Leading the execution of the AMT-061 HOPE-B pivotal study, including the activation of all clinical sites, the completion of patient enrollment and the exceeding of target patient dosing. |
| | | | |
Alexander Kuta | | Exceeded goals | | Leading the preparation, submission and FDA clearance of our Investigational New Drug application for AMT-130, as well as leading the successful realignment of the functions of our operations team beginning in August 2019. |
32
Table of Contents
Sander van Deventer | | Exceeded goals | | Leading the research of new liver-directed and CNS gene therapy product candidates, executing on a robust scientific communications program, completing proof-of-concept in a diseased animal model for our Fabry disease gene therapy product candidate and leading the realignment of our product development functions beginning in August 2019. |
The combination of this company-wide corporate performance and individual performance resulted in the following awards in respect of 2019 performance:
Named Executive Officer | | Actual Bonus | | Actual Bonus
(% of salary) | | Actual Bonus
(% of target) | |
Matthew Kapusta | | $ | 329,725 | | 60 | % | 109 | % |
Robert Gut | | $ | 185,951 | | 43 | % | 108 | % |
Alexander E. Kuta | | $ | 179,657 | | 44 | % | 110 | % |
Sander van Deventer | | $ | 182,503 | | 43 | % | 108 | % |
2019 Long-Term Incentives (Equity Awards)Incentive Awards
The Company’s 2014 Restated Plan provides that the Board may grant equity awards to senior managers.its employees. These grants include annual and periodic equity awards linked to continued employment and, at the Board’s discretion, the achievement of certain performance targets. Such grants as they apply to our named executive officers are fully described below. Pursuant to the 2014 Restated Plan, senior managersemployees may be granted options, restricted share units or performance share units (PSUs). PSU grantsunits. By awarding long-term incentive awards via a combination of different vehicles, the Compensation Committee can balance the objectives of driving sustainable long-term performance and shareholder value creation, encouraging retention while remaining market competitive.
The Company adopted an employee share purchase plan (the “Purchase Plan”) at the 2018 Annual General Meeting. The Purchase Plan is designed to allow eligible employees of the Company and its designated subsidiaries to purchase discounted Ordinary Shares at designated intervals through their accumulated payroll deductions. The provisions of the Purchase Plan are intended to satisfy the requirements of Section 423 of the U.S. Internal Revenue Code of 1986, as amended, with respect to U.S. participants. Favorable tax treatment is available for U.S. tax residents participating in a plan that qualifies under Section 423.
Awards are generally made annually in the first calendar quarter, taking into account impact on achieving our corporate goals, performance in the prior year and market data for the compensation peer group. The key features of each award type are as follows:

| · Options vest over a period of four years, with 25% of options granted becoming exercisable on the first anniversary, with the remaining options becoming exercisable pro-rata on a quarterly basis over the remaining three years. · Awards expire after ten years. · Share options cannot be repriced, reset, or exchanged for cash if underwater without shareholder approval. |
33
Table of Contents

| · Restricted Share Units vest pro-rata on an annual basis over three years. · Dividends do not accrue until shares are free from restrictions, unless expressly stated in the applicable award agreement. · Shares are issued to the participant upon vesting of the award, but may be subject to a nondiscretionary sale of a portion of the shares to cover tax withholding requirements. |
| |

| · Performance Share Units vest after three years subject to pre-established performance conditions. · The performance conditions are determined by the Board, and have historically been consistent with those established on a company-wide basis under the short-term incentive plan in the year of grant. · The payout range is 0%-150% of the target award. · Dividends do not accrue until shares are free from restrictions, unless expressly stated in the applicable award agreement. · Shares are issued to the participant upon vesting of the award, but may be subject to a sale of a portion of the shares to cover tax withholding requirements. |
Target equity awards are approved each year by the Compensation Committee, based on a combination of factors including overall corporate achievement, individual performance, granting history in prior years, impact on share utilization and dilution, impact of the individual on achieving the Company’s corporate goals, market practices and other relevant factors. In determining and approving award values, the Compensation Committee reviews data for our peer group and the overall total compensation of our executive officers. In light of the overall corporate performance and individual achievement in 2018, our Compensation Committee recommended that the Board grant long-term incentive equity awards that were commensurate with the 62.5th percentile of our peer group. Accordingly, target equity awards for named executive officers other than our CEO were approved at a level of approximately 175% to 270% of 2018 base salary, and target equity awards for our CEO were approved at a level of 567% of 2018 base salary.
Based on the terms of his employment agreement, Dr. Gut’s target equity award for 2019 was pro-rated by 50%, reflecting his appointment in August 2018.
In establishing the mix of long-term incentives to award our named executive officers, the Compensation Committee referenced market data for our peers, which found that most competitors grant awards in either stock options or a combination of stock options and restricted stock units. To further enhance the alignment of executive interests with the achievement of our corporate objectives, the Committee determined that it was appropriate for a portion of the awards to be linked to specific performance criteriain the form of performance stock units, accepting that this would differentiate uniQure from typical market practice.
In 2019, equity awards had the following target mix based on fair values determined as determinedof January 14, 2019:
34
Table of Contents
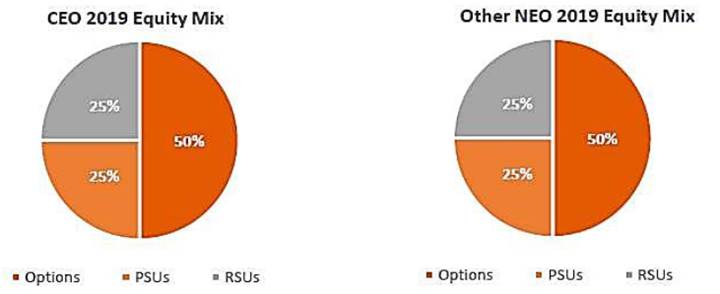
Employee Share Purchase Plan
The Employee Share Purchase Plan is designed to allow eligible employees of uniQure and its designated subsidiaries to purchase discounted Ordinary Shares at designated intervals through their accumulated payroll deductions. The purpose of the Plan is to provide employees with a convenient method to invest in uniQure Ordinary Shares which will increase the equity stake of our employees and will benefit shareholders by aligning more closely the interests of our participating employees with those of our shareholders. We believe that this will help to motivate and retain highly qualified employees.
Under the Plan, the number of Ordinary Shares initially reserved for issuance was 150,000. The purchase price of the Ordinary Shares acquired on each purchase date will be the lesser of (a) 85% of the closing price of an Ordinary Share on the first day of the offering period or (b) 85% of the closing price of an Ordinary Share on the purchase date.
CEO Pay Ratio
Under Item 402(u) of Regulation S-K adopted by the BoardSEC pursuant to the Dodd-Frank Wall Street Reform and willConsumer Protection Act of 2010, we are required to disclose the ratio of the annual total compensation of our Chief Executive Officer to the annual total compensation of our median compensated employee, excluding our CEO.
Matthew Kapusta (a) | | $ | 4,719,956 | |
Median Employee 2019 Annual Total Compensation | | $ | 122,079 | |
CEO to Median Employee Pay Ratio | | 39 to 1 | |
(a) This annual total compensation is the Total Compensation from the Summary Compensation Table.
Methodology
Our methodology for determining our CEO pay ratio relies on estimates and assumptions calculated in a manner consistent with SEC rules and guidance.
Determination of Employee Population
For this 2019 CEO pay ratio disclosure, we have used the median employee analysis that we conducted last year for the 2018 CEO pay ratio disclosure. We believe there has been no change in our employee population or employee compensation arrangements that would significantly affect the pay ratio disclosure for 2019. For the 2018 disclosure, we determined our global employee population as of the December 31, 2018, including full-time, part-time, seasonal and temporary workers, other than our CEO. As of December 31, 2019, we had a total of 248
35
Table of Contents
employees, 116 of whom were based in Amsterdam, The Netherlands, and 132 in Lexington, Massachusetts. As of September 30, 2020, we had a total of 314 employees, 152 of whom were based in Amsterdam, The Netherlands, and 162 in Lexington, Massachusetts.
Calculating Median Employee Compensation
To identify the median employee in 2018, we used base salary as our consistently applied compensation measure (“CACM”), which we obtained from our payroll records across our global employee population. We used the total wages earned in that calendar year, adjusted the pay of employees in Europe from Euros to U.S. Dollars using the average exchange rate that we applied in our financial statements, and, where applicable, pro-rated the annualized base salary of any non-hourly, part-time employees to reflect the full-time actual salary being earned. The employee that was determined to be earnedour median employee for purposes of our 2018 CEO pay ratio disclosure is no longer employed by uniQure. Therefore, we have selected a similarly compensated employee for this 2019 disclosure. In the median employee analysis, the median fell between two similarly compensated employees. Thus, for this 2019 disclosure, we have selected as the median employee the other of those two employees.
Based upon the comparison using the CACM, we determined that the total annual compensation of our median employee was $122,079 as of December 31, 2019.
Our CEO to median employee pay ratio is 39 to 1.
The SEC’s rules for identifying the median compensated employee and calculating the pay ratio based on that employee’s annual total compensation allow companies to adopt a variety of methodologies, to apply certain exclusions, and to make reasonable estimates and assumptions that reflect their employee populations and compensation practices. As a result, the actual achievement of this specific criteria during the performance period, typically one year following the date of grant (known as the performance period), as determinedpay ratio reported by the Board. The vesting period applicableother companies, including our compensation peer group, may not be comparable to the PSUs will be set by the Board at the time of grantpay ratio reported above, as other companies have different employee populations and is typically three years following the date of the grant. Upon vesting of the PSUs, shares are automatically granted to the grantee.compensation practices and may utilize different methodologies, exclusions, estimates and assumptions in calculating their own pay ratios.
Employment Agreements
Matthew Kapusta
Prior to becoming our chief executive officer,Chief Executive Officer, Mr. Kapusta served as our chief financial officer.Chief Financial Officer. On December 9, 2014, the Company entered into an employment agreement with Mr. Kapusta for the role of chief financial officerChief Financial Officer (the “Kapusta CFO Agreement”). On October 19, 2017 (the “First Kapusta Amendment, March 14, 2017 (the “Second Kapusta Amendment”) and October 26, 2017 (the “Third Kapusta Amendment,” together with the First Kapusta Amendment and the Second Kapusta Amendment, the “Kapusta Agreement Amendments”), the Company entered into an amendmentamendments to the Kapusta CFO Agreement in connection with his new role as chief executive officerChief Executive Officer (the “Kapusta Agreement Amendment”, the Kapusta CFO Agreement as amended by the Kapusta Amendment Agreement Amendments being the “Kapusta Employment Agreement”). The Kapusta Employment Agreement provides that Mr. Kapusta will earn a base salary equal to $450,000 per year effective January 1, 2017, plus reimbursement of expenses incurred on the Company’s behalf. Mr,Effective January 1, 2020, Mr. Kapusta’s base salary was increased to $566,500 per year. Mr. Kapusta is also eligible for an annual performance bonus with a target for 20172020 of 50%60% of his base salary and a grant of restricted share units as further described in the Kapusta Employment Agreement. The termination provisions orof the Kapusta Employment Agreements are further discussed below. The term of the Kapusta Employment Agreement iswill run through December 31, 2018.2020 or until terminated by either us or by Mr. Kapusta. A copy of the Kapusta CFO Agreement wasis filed as Exhibit 10.6 to the Company’s Annual Report on Form 10-K as filed with the SEC on March 15, 2017. A copy of the Second Kapusta Agreement Amendment wasis filed as Exhibit 10.7 to the Company’s Annual Report on Form 10-K as filed with the SEC on March 15, 2017. A copy of the Third Kapusta Amendment is filed as Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q filed with the SEC on November 1, 2017. The foregoing are not complete descriptions of the Kapusta Employment Agreement doand are qualified in their entirety by reference to the full text of such agreement.
36
Table of Contents
Robert Gut
Dr. Gut entered into an employment agreement with the Company on August 20, 2018 for the role of Chief Medical Officer (the “Gut Employment Agreement”). The Gut Employment Agreement was terminated on October 14, 2020, when Dr. Gut resigned from the Company and from the Board pursuant to a Separation Agreement dated August 25, 2020.
The Gut Employment Agreement provided that Dr. Gut will receive a base salary of $425,000 per year, subject to review at the sole discretion of the Company and a discretionary bonus of up to 40% of annual base salary (with any such bonus for 2018 being pro-rated for length of service). Under the Gut Employment Agreement, Dr. Gut was also entitled to expenses and reimbursements, including reimbursement for certain relocation fees incurred to a maximum of $75,000 for up to one year. He was also entitled to a grant of 35,000 restricted stock units and an option to purchase 70,000 ordinary shares in the Company each pursuant to the Company’s equity incentive plan and would be eligible for future grant awards. Effective November 1, 2019, the Gut Employment Agreement was amended to provide an additional relocation benefit for reimbursement of certain relocation fees incurred to a maximum of $50,000 for up to one year from the date of the amendment. Effective March 1, 2020, the Gut Employment Agreement was amended and restated to, among other things, provide additional severance benefits, including an increase in severance payments: (1) to 150% of base salary plus target bonus and COBRA coverage for 18 months for a termination in association with a change of control of the Company and (2) to 100% of base salary plus target bonus and COBRA coverage for 12 months for other qualifying terminations; as well as a pro-rata cash bonus for the current (interrupted) year in which any qualifying termination occurs. In March 2020, Dr. Gut received a letter (the “Gut 2020 Letter”), which provided that his 2020 base salary would be $442,535 and his 2019 bonus would be $185,951. The Gut 2020 Letter also provided that Dr. Gut would be entitled to participate in the 2020 equity grants of 12,380 restricted share units and an option to purchase 21,719 ordinary shares in the Company each pursuant to the Company’s equity incentive plan. The termination provisions of the Gut Employment Agreement are further discussed below. The Gut Employment Agreement was to continue in force from year to year unless terminated in accordance with its terms. A copy of the Gut Employment Agreement is filed as Exhibit 10.50 to the Company’s Annual Report on Form 10-K filed with the SEC on March 2, 2020. The foregoing are not purport to be complete descriptions of the Gut Employment Agreement and are qualified in their entirety by reference to the full text of such agreement.
Daniel SolandAlexander E. Kuta
On December 17, 2015, the CompanyDr. Kuta entered into an employment agreement with Mr. Solandthe Company on January 23, 2017 for the role of chief executive officerSenior Vice President, Regulatory Affairs (the “Soland“Kuta Employment Agreement”). In September 2016, Mr. Soland resigned from his role as chief executive officer. In connection with his resignation, he executed an acknowledgement and release of claims (the “Soland Release”) which stated he resigned without Good Reason (as defined below) and that he was entitled to no severance or other benefit other than Accrued Benefits (as defined below), to which the Company was entitled to a right of offset. He also released us from all claims related to the termination of his employment.
The SolandKuta Employment Agreement providedprovides that Mr. Soland was to earnDr. Kuta will receive a base salary equal to $500,000of $375,000 per year, (to be reviewed annually bysubject to review at the Boardsole discretion of the Company and a discretionary bonus of up to 35% of annual base salary (with any such bonus for readjustment), plus reimbursement2017 being pro-rated for length of service). Under the Kuta Employment Agreement, Dr. Kuta is also entitled to expenses incurred on the Company’s behalf. Mr. Solandand reimbursements. He was also entitled to a grant of an annual performanceoption to purchase 150,000 ordinary shares in the Company pursuant to the Company’s equity incentive plan and would be eligible for future grant awards. Effective August 20, 2019, the Kuta Employment Agreement was amended and restated to, among other things, provide for a promotion to the position of Executive Vice President, Operations, a base salary of $429,646 annually, an additional equity grant of 15,000 restricted share units pursuant to the Company’s equity incentive plan, and additional severance benefits, including an increase in severance payments: (1) to 150% of base salary plus target bonus and COBRA coverage for 18 months for a termination in association with a change of control of the Company and (2) to 100% of base salary plus target bonus and COBRA coverage for 12 months for other qualifying terminations; as well as a pro-rata cash bonus for the current (interrupted) year in which any qualifying termination occurs.
In March 2020, Dr. Kuta received a letter (the “Kuta 2020 Letter”), which provides that his 2020 base salary will be $436,091 and his 2019 bonus will be $179,657. The Kuta 2020 Letter also provides that Dr. Kuta will be entitled to participate in the 2020 equity grants of 50% of his base salary.12,380 restricted share units and an option to purchase 21,719 ordinary shares in the Company each pursuant to the Company’s equity incentive plan. The termination provisions of the SolandKuta Employment Agreement are further discussed below. The Kuta Employment Agreement is to continue in force from year to year unless terminated in accordance with its terms. A copy of the Kuta Employment Agreement is filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on September 20, 2019. The
37
Table of Contents
foregoing are not complete descriptions of the Gut Employment Agreement and are qualified in their entirety by reference to the full text of such agreement.
Charles RichardSander van Deventer
On June 24, 2015, the CompanyDr. van Deventer entered into an employment agreement with Dr. Richardthe Company on August 7, 2017 for the role of senior vice president, neuroscience research and developmentChief Scientific Officer (the “Richard“van Deventer Employment Agreement”). As of December 31, 2016,The van Deventer Employment Agreement was terminated on September 14, 2020, when Dr. Richard’s employment withvan Deventer resigned from the Company ended. In connection with the end of his employment, he entered intopursuant to a separation agreement with the Company (the “Richard Separation Agreement”) which provided for certain compensation related to his separation from employment as described below. He also released us from all claims related to the termination of his employment.Agreement dated August 25, 2020.
The Richardvan Deventer Employment Agreement providesprovided that Dr. Richard was to earnvan Deventer would receive a base salary equal to $326,500of €200,000 per year, (to be reviewed
annually bysubject to review at the Company’s chief executive officersole discretion of the Company and a discretionary bonus of up to 40% of his annual base salary (with any such bonus for readjustment), plus reimbursement2017 being pro-rated for length of service). Under the van Deventer Employment Agreement, Dr. van Deventer was also entitled to expenses incurred on the Company’s behalf. Dr. Richardand reimbursements. He was also entitled to a discretionarygrant of an option to purchase 150,000 ordinary shares in the Company pursuant to the Company’s equity incentive plan and would be eligible for future grant awards. Effective August 20, 2019, the van Deventer Employment Agreement was amended and restated to, among other things, provide for a promotion to the position of Executive Vice President, Product and Research Development, a base salary of €348,000 annually, an additional equity grant of 15,000 restricted share units pursuant to the Company’s equity incentive plan, and additional severance benefits, including an increase in severance payments: (1) to 150% of base salary plus target bonus for a termination in association with a change of control of the Company and (2) to 100% of base salary plus target bonus for other qualifying terminations; as well as a pro-rata cash bonus for the current (interrupted) year in which any qualifying termination occurs. In March 2020, Dr. van Deventer received a letter (the “van Deventer 2020 Letter”), which provided that his 2020 base salary would be €353,220 and his 2019 bonus would be €111,989. The van Deventer 2020 Letter also provided that Dr. van Deventer would be entitled to participate in the 2020 equity grants of 35% of his base salary.12,380 restricted share units and an option to purchase 21,719 ordinary shares in the Company each pursuant to the Company’s equity incentive plan. The termination provisions of the Richardvan Deventer Employment Agreement were superseded by the Richard Separation Agreement, which isare further discussed below.
Dr. Richard The van Deventer Employment Agreement is considered one of our named executive officers becauseto continue in force from year to year unless terminated in accordance with its terms. A copy of the compensation received in connectionvan Deventer Employment Agreement is filed as Exhibit 10.2 to the Company’s Current Report on Form 8-K filed with the Richard Separation Agreement.
Deya Corzo
On August 1, 2015,SEC on September 20, 2019. The foregoing are not complete descriptions of the Company entered into an employment agreement with Dr. Corzo for the role of senior vice president, liver/metabolic therapeutics leader (the “Corzovan Deventer Employment Agreement”). As of December 31, 2016, Dr. Corzo’s employment with the Company ended. In connection with the end of her employment, she entered into a separation agreement with the Company (the “Corzo Separation Agreement”) which provided for certain compensation related to her separation from employment as described below. She also released us from all claims relatedAgreement and are qualified in their entirety by reference to the terminationfull text of her employment.
The Corzo Employment Agreement provides that Dr. Corzo was to earn a base salary equal to $342,825 per year (to be reviewed annually by the Company’s chief executive officer for readjustment), plus reimbursement of expenses incurred on the Company’s behalf. Dr. Corzo was also entitled to a discretionary bonus with a target of 35% of her base salary. The termination provisions of the Corzo Employment Agreement were superseded by the Corzo Separation Agreement, which is further discussed below.
Dr. Corzo is considered one of our named executive officers because of the compensation received in connection with the Corzo Separation Agreement.such agreement.
Other Executive Compensation Policies
Tax and Accounting Considerations for named executive officer subject to US tax legislation
Prior to the passage of the Tax Cuts and Jobs Act of 2017, Section 162(m) of the Internal Revenue Code of 1986, as amended (the “Internal Revenue Code”), had generally disallowsdisallowed a tax deduction for compensation in excess of $1.0 million paid to oura company’s named executive officers, whose compensation is required to be disclosed to our Shareholders under the Exchange Act. Qualifyingother than its chief financial officer. Historically, qualifying performance-based compensation iswas not subject to the deduction limitation if specified requirements arewere met. The Company seeks to structureHowever, effective for taxable years beginning after December 31, 2017, the exemption for qualified performance-based portioncompensation from the deduction limitation of any executive compensation package to comply with exemptions in Section 162(m) sohas been repealed, such that compensation paid to our NEOs in excess of $1 million will not be deductible unless it qualifies for the limited transition relief applicable to certain compensation remains tax deductible to the Company. However, the Compensation Committee may recommend to the Board compensation payments that do not comply with the exemptionsarrangements in Section 162(m) when it believes that such payments are appropriate to attract and retain executive talent.certain arrangements place as of November 2, 2017.
“Nonqualified deferred compensation” is required by Section 409A of the Internal Revenue Code to be paid under plans or arrangements that satisfy certain statutory requirements regarding timing of deferral elections, timing of payments and certain other matters. Employees and service providers who receive compensation that fails to satisfy these requirements may be subject to accelerated income tax liabilities, a 20% excise tax, penalties and interest on their compensation under such plans. The Company seeks to design and administer our compensation and benefits plans and arrangements for all of our employees and service providers, including our named executive officers, to keep them either exempt from or in compliance with the requirements of Section 409A.
38
Table of Contents
Sections 280G and 4999 of the Internal Revenue Code impose certain adverse tax consequences on compensation treated as excess parachute payments. An executive is treated as having received excess parachute payments if such executive receives compensatory payments or benefits that are contingent on a change in control, and the aggregate amount of such payments and benefits equal or exceeds three times the executive’s base salary amount. The portion of the payments and benefits in excess of one times base salary amount are treated as excess parachute payments and are subject to a 20% excise tax, in addition to any applicable federal income and employment taxes.
Deferred Compensation and Retirement Plans
The Company operates a qualified 401(k) Plan for all employees at its Lexington facility in the USA. The uniQure, Inc. 401(k) Plan is an employee contribution plan only, and there are no employer contributions currently being made. The uniQure Inc. 401(k) Plan offers both a before tax and after tax (Roth) component, which are subject to IRS statutory limits for each calendar year.
The Company operates a defined contribution pension plan for all employees at its Amsterdam facility in the Netherlands, which is funded by the GroupCompany through payments to an insurance company.
Equity Incentive Plan
The 2014 Restated Plan enables the Board to among others grant options, Restricted StockShare Units (RSUs) and PSUs. The purpose of the 2014 Restated Plan is to advance the interests of the Company’s shareholders by enhancing the Company’s ability to attract, retain and motivate persons who are expected to make important contributions to the group and by providing such persons with equity ownership opportunities and performance-based incentives that are intended to better align the interests of such persons with those of the Company’s shareholders.
The terms of the PSUs are further discussed above. For both RSUs and PSUs, the shares are automatically issued to the grantee upon the vesting of the award.
Under the 2014 Restated 2014 Plan, the maximum number of Ordinary Shares available is currently limited to 5,601,471.8,601,471. As of July 1, 2017, 1,575,764September 30, 2020, 2,230,615 Ordinary Shares remainedremain available for grant under the 2014 Restated 2014 Plan.
Employee Share Purchase Plan
The ESPP is designed to allow eligible employees of the Company and its designated subsidiaries to purchase discounted Ordinary Shares at designated intervals through their accumulated payroll deductions. The purpose of the ESPP is to provide employees with a convenient method to invest in the Company’s Ordinary Shares which will increase the equity stake of the Company’s employees and will benefit shareholders by aligning more closely the interests of participating employees with those of the Company’s shareholders. The Company believes that this will help to motivate and retain highly qualified employees.
Under the ESPP, the number of Ordinary Shares initially reserved for issuance is 150,000. The purchase price of the Ordinary Shares acquired on each purchase date will be the lesser of (a) 85% of the closing price of an Ordinary Share on the first day of the offering period or (b) 85% of the closing price of an Ordinary Share on the purchase date. As of September 30, 2020, 134,925 Ordinary Shares remain available for issuance under the ESPP.
Role of Executive OfficersOfficer in Determining Executive Compensation
The Compensation Committee and Board approve all compensation decisions related to our named executive officers.Named Executive Offices. Such decisions by the Compensation Committee regarding compensation were made independently from our named executive officers.
Stock Ownership Requirements and Hedging Policies
Currently, the Company does not have any formal stock ownership requirements or any specific hedging policies related to stock ownership.
Risk Considerations
The Compensation Committee annually evaluates whether there are potential risks arising from the Company’s compensation policies and practices. Based on such evaluation, the Compensation Committee believes that the
39
Table of Contents
Company’s compensation policies and practices do not encourage executives to take excessive risks because the various elements of the Company’s executive compensation policies and practices diversify the risks associated with any single element of the executive’s compensation. Instead, the elements of the Company’s executive compensation policy are, collectively, designed to achieve the Company’s annual and long-term corporate objectives and strategies.
40
Table of Contents
SUMMARY COMPENSATION TABLE
The following table summarizes the annual compensation paid to our named executive officers for the twothree fiscal years ended December 31, 20162019, 2018 and 2015.2017.
| | | | | | | | Stock | | Option | | All Other | | | |
Name and | | | | Salary | | Bonus | | Awards | | Awards | | Compensation | | | |
Position | | Year | | ($) | | ($) | | ($) | | ($) | | ($) | | Total ($) | |
| | | | | | | | | | | | | | | |
Matthew Kapusta, Chief Executive Officer and interim Chief Financial Officer (1) | | 2016 | | 379,996 | | 142,325 | | 111,129 | | 574,938 | | 29,230 | | 1,237,640 | |
| | | | | | | | | | | | | | | |
| | 2015 | | 350,000 | | 140,000 | | — | | 331,294 | | 175,000 | | 996,294 | |
| | | | | | | | | | | | | | | |
Daniel Soland, Chief Executive Officer (2) | | 2016 | | 376,730 | | — | | — | | (154,494 | ) | 28,252 | | 250,488 | |
| | | | | | | | | | | | | | | |
| | 2015 | | — | | — | | — | | 154,494 | | — | | 154,494 | |
| | | | | | | | | | | | | | | |
Charles Richard, SVP, Research and Development Neuroscience (3) | | 2016 | | 352,620 | | 103,814 | | 69,120 | | 789,000 | | 364,110 | | 1,678,664 | |
| | | | | | | | | | | | | | | |
| | 2015 | | 196,813 | | 53,537 | | — | | 394,500 | | 11,000 | | 655,850 | |
| | | | | | | | | | | | | | | |
Deya Corzo, SVP, Therapeutic area head Liver/Metabolism (4) | | 2016 | | 353,110 | | 123,589 | | 129,600 | | 90,919 | | 375,110 | | 1,072,328 | |
| | | | | | | | | | | | | | | |
| | 2015 | | 345,964 | | 119,989 | | — | | 199,813 | | 22,000 | | 687,766 | |
Name | | Year | | Salary (1)
($) | | Stock
Award(2)
($) | | Option
Awards(2)
($) | | Non-Equity
Incentive Plan
Compensation(3)
($) | | Medicare
benefit
($) | | All other
compensation
($) | | Total
($) | |
Matthew Kapusta | | 2019 | | 547,885 | | 2,856,387 | | 954,938 | | 329,725 | | 22,620 | | 8,400 | | 4,719,956 | |
| | 2018 | | 501,923 | | 2,388,508 | | 501,923 | | 350,000 | | 23,596 | | 8,100 | | 4,095,328 | |
| | 2017 | | 468,109 | | 2,612,217 | | 560,496 | | 257,624 | | 23,745 | | 7,343 | | 3,929,535 | |
Robert Gut | | 2019 | | 532,816 | | 631,842 | | 570,499 | | 185,951 | | 19,946 | | 8,400 | | 1,949,454 | |
| | 2018 | (4) | 177,751 | | 132,123 | | 158,776 | | 81,768 | | 7,827 | | 3,923 | | 562,168 | |
Alexander E. Kuta | | 2019 | | 405,912 | | 466,787 | | 275,639 | | 179,657 | | 16,638 | | 8,400 | | 1,353,032 | |
| | 2018 | | 387,736 | | 166,509 | | 183,641 | | 182,503 | | 16,648 | | 8,100 | | 1,223,049 | |
| | 2017 | (5) | 353,365 | | — | | 110,554 | | 141,610 | | 15,317 | | 8,100 | | 628,946 | |
Sander van Deventer | | 2019 | | 292,272 | | 424,694 | | 335,186 | | 125,383 | | — | | 13,386 | | 1,190,920 | |
| | 2018 | | 252,651 | | 93,242 | | 230,399 | | 130,112 | | — | | 13,771 | | 720,175 | |
| | 2017 | (6) | 145,139 | | 28,879 | | 69,453 | | 42,682 | | — | | 5,659 | | 242,772 | |
(1) Mr. Kapusta served Salary is determined based on actual salary during the fiscal years 2017 - 2019.
(2) The value of stock awards and stock options as reported in their respective columns is calculated using the grant date accounting fair value determined in accordance with Accounting Standards Codification 718, Compensation-Stock Compensation (“ASC 718”). Amounts reflected in the stock awards column are comprised of the accounting value of both the time-vested RSUs and PSUs granted in the years reflected. For assumptions and estimates used in determining these values, see Management’s Discussion and Analysis of Financial Condition and Results of Operations — Share-based Payments and Note 2.3.18 of the Consolidated Financial Statements in our Chief Financial Officer since2019 Annual Report on Form 10-K.
(3) These amounts reflect the annual cash bonus awards granted to the named executive officers pursuant to the Company’s Short- term Incentive program.
(4) Dr. Gut’s employment commenced on August 20, 2018.
(5) Dr. Kuta’s employment commenced on January 2015 and was appointed as our interim Chief Executive Officer25, 2017.
(6) Dr. van Deventer’s employment commenced on September 2016. He was appointed Chief Executive Officer on December 14, 2016 and has served as interim Chief Financial Officer since then.August 7, 2017.
(2) Mr. Soland served as our Chief Executive Officer from December 2015 until September 2016.41
(3) Dr. Richard’s employment ended effective December 31, 2016.
(4) Dr. Corzo’s employment ended effective December 31, 2016.Table of Contents
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR END (2016)2019
The following table contains information concerning exercisable stock options with respect to our Ordinary Shares, RSUs and PSUs granted to our named executive officers that were outstanding onas of December 31, 2016.2019.
| | | | Option Awards (1) | | Stock Awards (2) | |
Name | | Type of Equity
Award | | Number of
Securities
Underlying
Unexercised
Options
(#) Exercisable | | Number of
securities
underlying
unexercised
options
(#)
Unexercisable | | Equity
incentive plan
awards:
Number of
securities
underlying
unexercised
unearned
options
(#) | | Option
Exercise
Price
($) | | Option
Expiration
Date | | Number of
Shares or
Units of Stock
That Have
Not Yet
Vested (#)(3) | | Market
Value of
Shares or
Units of
Stock That
Have Not
Vested ($) | | Equity Incentive
Plan Awards:
Number of
Unearned
Shares, Units or
Rights That
Have Not Yet
Vested (#) (4) | | Equity Incentive
Plan Awards:
Market or Payout
Value of Unearned
Shares Units or
Other Rights That
Have Not Vested ($) | |
Matthew Kapusta, Chief Executive Officer and Chief Financial Officer (5) | | Options (6) | | 56,250 | | 43,750 | | — | | 14.71 | | 2025 | | — | | — | | — | | — | |
| Options (7) | | 43,750 | | 56,250 | | — | | 23.60 | | 2025 | | — | | — | | — | | — | |
| Options (8) | | 0 | | 100,000 | | — | | 7.53 | | 2026 | | — | | — | | — | | — | |
| RSUs | | — | | — | | — | | — | | — | | — | | — | | — | | — | |
| PSUs (9) | | — | | — | | — | | — | | 2026 | | 23,064 | | 129,158.40 | | 61,560 | | 344,736 | |
| | | | | | | | | | | | | | | | | | | | | |
Daniel Soland, Chief Executive Officer (10) | | Options | | — | | — | | — | | — | | — | | — | | — | | — | | — | |
| RSU’s | | — | | — | | — | | — | | — | | — | | — | | — | | — | |
| PSUs | | — | | — | | — | | — | | — | | — | | — | | — | | — | |
| | | | | | | | | | | | | | | | | | | | | |
Charlie Richards, SVP, Research and Development Neuroscience (11) | | Options (12) | | 62,500 | | — | | — | | 18.21 | | 2017 | | — | | — | | — | | — | |
| | RSUs | | — | | — | | — | | — | | — | | — | | — | | — | | — | |
| | PSUs (13) | | — | | — | | — | | — | | 2026 | | — | | — | | 12,000 | | 67,200 | |
| | | | | | | | | | | | | | | | | | | | | |
Deya Corzo, SVP, Therapeutic area head Liver/Metabolism (14) | | Options (15) | | 15,625 | | — | | — | | 9.35 | | 2017 | | — | | — | | — | | — | |
| Options (16) | | 15,625 | | — | | — | | 23.60 | | 2017 | | — | | — | | — | | — | |
| RSUs | | — | | — | | — | | — | | — | | — | | — | | — | | — | |
| PSUs (17) | | — | | — | | — | | — | | 2026 | | — | | — | | 22,500 | | 126,000 | |
| | Option Awards (1) | | Stock Awards (2) | |
Name | | Type of
Equity
Award | | Number of
Securities
Underlying
Exercised
Options
(#) | | Number of
Securities
Underlying
Unexercised
Options
Unexercisable
(#) | | Equity
Incentive Plan
Awards:
Number of
Securities
Underlying
Unexercised
Unearned
Options
(#) | | Option
Exercise
Price
($) | | Option
Expiration
Date | | Number of
Shares or
Units of
Stock That
Have Not
Yet Vested
(3)
(#) | | Market
Value of
Shares or
Units of
Stock That
have Not
Vested (#) | | Equity
Incentive
Plan
Awards:
Number of
Unearned
Shares,
Units or
Rights That
Have Not
Yet Vested
(4)
(#) | | Equity
Incentive
Plan
Awards:
Market
or
Payout
Value of
Unearned
Shares
Units or
Other
Rights
That
Have
Not
Vested
($) | |
Matthew Kapusta | | Options | | 100,000 | | — | | — | | 14.71 | | 2025 | | — | | — | | — | | — | |
| | Options | | 100,000 | | — | | — | | 23.60 | | 2025 | | — | | — | | — | | — | |
| | Options | | 73,911 | | 18,750 | | — | | 7.53 | | 2026 | | — | | — | | — | | — | |
| | Options | | 120,312 | | 54,688 | | — | | 6.22 | | 2027 | | — | | — | | — | | — | |
| | Options | | 36,602 | | 47,061 | | — | | 19.39 | | 2028 | | — | | — | | — | | — | |
| | Options | | — | | 83,362 | | — | | 31.71 | | 2029 | | — | | — | | — | | — | |
| | RSUs(3) | | — | | — | | — | | — | | — | | 20,916 | | 1,498,841 | | — | | — | |
| | RSUs(4) | | — | | — | | — | | — | | — | | 25,425 | | 1,821,956 | | — | | — | |
| | PSUs(7) | | — | | — | | — | | — | | — | | 209,625 | | 15,021,728 | | — | | — | |
| | PSUs(8) | | — | | — | | — | | — | | — | | 43,924 | | 3,147,594 | | — | | — | |
| | PSUs(9) | | — | | — | | — | | — | | — | | 27,713 | | 1,985,914 | | — | | — | |
Robert Gut | | Options | | 5,000 | | 5,000 | | — | | 35.40 | | 2028 | | — | | — | | — | | — | |
| | Options | | 21,875 | | 48,125 | | — | | 39.97 | | 2028 | | — | | — | | — | | — | |
| | Options | | — | | 16,877 | | — | | 31.71 | | 2029 | | — | | — | | — | | — | |
| | RSUs(5) | | — | | — | | — | | — | | — | | 23,334 | | 1,672,114 | | — | | — | |
| | RSUs(4) | | — | | — | | — | | — | | — | | 5,147 | | 368,834 | | — | | — | |
| | PSUs(9) | | — | | — | | — | | — | | — | | 5,610 | | 402,013 | | — | | — | |
Alexander E. Kuta | | Options | | 55,125 | | 46,875 | | — | | 5.31 | | 2027 | | — | | — | | — | | — | |
| | Options | | 10,483 | | 13,479 | | — | | 19.39 | | 2028 | | — | | — | | — | | — | |
| | Options | | — | | 19,883 | | — | | 31.71 | | 2029 | | — | | — | | — | | — | |
| | RSUs(3) | | — | | — | | — | | — | | — | | 5,991 | | 429,315 | | — | | — | |
| | RSUs(4) | | — | | — | | — | | — | | — | | 6,064 | | 434,546 | | — | | — | |
| | RSUs(6) | | — | | — | | — | | — | | — | | 15,000 | | 1,074,900 | | — | | — | |
| | PSUs(8) | | — | | — | | — | | — | | — | | 12,580 | | 901,483 | | — | | — | |
| | PSUs(9) | | — | | — | | — | | — | | — | | 6,610 | | 473,673 | | — | | — | |
Sander van Deventer | | Options | | 5,000 | | — | | — | | 18.21 | | 2026 | | — | | — | | — | | — | |
| | Options | | 11,000 | | — | | — | | 5.37 | | 2027 | | — | | — | | — | | — | |
| | Options | | 84,375 | | 65,625 | | — | | 8.49 | | 2027 | | — | | — | | — | | — | |
| | Options | | 9,094 | | 11,694 | | — | | 19.39 | | 2028 | | — | | — | | — | | — | |
| | Options | | — | | 18,325 | | — | | 31.71 | | 2029 | | — | | — | | — | | — | |
| | RSUs(3) | | — | | — | | — | | — | | — | | 5,197 | | 372,417 | | — | | — | |
| | RSUs(4) | | — | | — | | — | | — | | — | | 5,589 | | 400,508 | | — | | — | |
| | RSUs(6) | | — | | — | | — | | — | | — | | 15,000 | | 1,074,900 | | — | | — | |
| | PSUs(8) | | — | | — | | — | | — | | — | | 10,913 | | 782,026 | | — | | — | |
| | PSUs(9) | | — | | — | | — | | — | | — | | 6,092 | | 436,553 | | — | | — | |
(1) The option grants typically vest over four years; 25% on the anniversary of the grant date and in equal quarterly installmentsmonthly instalments thereafter.
(2) RSU and PSU awards are valued based on the closing stock price of the Company on December 30, 201631, 2019 ($5.60)
71.66).
(3) 2018 RSU awards granted on January 26, 2018, vest 100% on the first anniversary following1/3 after each of one year, two years and three years after the grant date.
42
Table of Contents
(4) 2019 RSU awards granted on January 25, 2019, vest 1/3 after each of one year, two years and three years after the grant date.
(5) RSU awards granted on September 18, 2018, vest 1/3 after each of one year, two years and three years after the grant date.
(6) RSU awards granted on September 17, 2019, vest 1/3 after each of one year, two years and three years after the grant date.
(7) 2017 PSU awards granted on January 27, 2017, were earned in December 2017 and vested on January 27, 2020.
(8) 2018 PSU awards granted on January 26, 2018, were earned in January 2019 and vest on January 26, 2021.
(9) PSU awards granted on January 25, 2019 vest three years following the date of the grant, subject to the achievement of performance metrics. The performance metrics were achieved and PSUs were earned on February 27, 2020.
(5) Mr. Kapusta served as our Chief Financial Officer since January 2015 and was appointed as our interim Chief Executive Officer on September 2016. He was appointed Chief Executive Officer on December 14, 2016 and has served as interim Chief Financial Officer since then.43
Table of Contents
(6) The grant date was January 2, 2015.GRANTS OF PLAN-BASED AWARDS FOR 2019
(7) The grant date was August 25, 2015.following table sets forth information relating to non-equity incentives awards granted pursuant to our Short-term Incentive program and equity awards granted pursuant to our Long-term Incentive program during the year ended December 31, 2019 to each of our named executive officers:
(8) The grant
| | | | | | Estimated Possible
Payouts under Non-Equity Incentive
Plan Awards(1) | | Estimated Future Payouts
Under Equity Incentive Plan
Awards (4) | | | | | | | | | |
Name | | Award | | Grant Dates | | Theshold
($) | | Target
($) | | Maximum
($) | | Threshold
($) | | Target
(#) | | Maximum
(#) | | All Other Stock
Awards:
Number of
shares of stock
or units# | | All Other
Stock
Awards:
Number of
Securities
underlying
Option # | | Exercise or
Base Price
of Option
Awards
($/sh) | | Grant Date
Fair Value
of Stock and
Option
Awards ($) | |
Matthew Kapusta | | IC(1) Option(2) | | — 01/25/19 | | 151,250 — | | 302,500 — | | 453,750 — | | —
— | | —
— | | —
— | | —
— | | — 83,362 | | — 31.71 | | — 1,564,705 | |
| | RSU(3) | | 01/25/19 | | — | | — | | — | | — | | — | | — | | 25,425 | | — | | — | | 982,168 | |
| | PSU(4) | | 01/25/19 | | — | | — | | — | | 0 | | 25,425 | | 38,138 | | 27,713 | | — | | — | | 1,435,811 | |
Robert Gut | | IC(1) | | — | | 85,929 | | 171,858 | | 257,788 | | — | | — | | — | | — | | — | | — | | — | |
| | Option(2) | | 01/25/19 | | — | | — | | — | | — | | — | | — | | — | | 16,877 | | 31.71 | | 316,781 | |
| | RSU(3) | | 01/25/19 | | — | | — | | — | | — | | — | | — | | 5,147 | | — | | — | | 198,829 | |
| | PSU(4) | | 01/25/19 | | — | | — | | — | | 0 | | 5,147 | | 7,721 | | 5,610 | | — | | — | | 290,654 | |
Alexander E. Kuta | | IC(1) | | — | | 81,886 | | 163,771 | | 245,657 | | — | | — | | — | | — | | — | | — | | — | |
| | Option(2) | | 01/25/19 | | — | | — | | — | | — | | — | | — | | — | | 19,883 | | 31.71 | | 373,204 | |
| | RSU(3) | | 01/25/19 | | — | | — | | — | | — | | — | | — | | 6,065 | | — | | — | | 234,252 | |
| | RSU(3) | | 09/17/19 | | — | | — | | — | | — | | — | | — | | 15,000 | | — | | — | | 728,100 | |
| | PSU(4) | | 01/25/19 | | — | | — | | — | | 0 | | 6,064 | | 9,096 | | 6,610 | | — | | — | | 342,464 | |
Sander van Deventer(5) | | IC(1) | | — | | 58,454 | | 116,909 | | 175,363 | | — | | — | | — | | — | | — | | — | | — | |
| | Option(2) | | 01/25/19 | | — | | — | | — | | — | | — | | — | | — | | 18,325 | | 31.71 | | 343,960 | |
| | RSU(3) | | 01/25/19 | | — | | — | | — | | — | | — | | — | | 5,589 | | — | | — | | 215,903 | |
| | RSU(3) | | 09/17/19 | | — | | — | | — | | — | | — | | — | | 15,000 | | — | | — | | 728,100 | |
| | PSU(4) | | 01/25/19 | | — | | — | | — | | 0 | | 5,589 | | 8,384 | | 6,0926 | | — | | — | | 315,627 | |
(1) Represents 2019 annual cash incentive awards granted under the Company’s Short-Term Incentive Plan. For additional information, please see “Compensation Discussion and Analysis—2019 Short-Term Incentive Plan”.
(2) Time-vested stock options granted under the Company’s 2014 Restated Plan. Grant date was September 29, 2016.values are determined in accordance with ASC Topic 718. See “Compensation Discussion and Analysis—2019 Long-term Incentive Awards”.
(3) Time-vested RSUs granted under the Company’s 2014 Restated Plan. Grant date values are determined in accordance with ASC Topic 718. See “Compensation Discussion and Analysis—2019 Long-term Incentive Awards”.
(4) Three-year PSUs granted in 2019 under the Company’s 2014 Restated Plan for the 2019-2021 performance period were earned on January 317, 2020. Grant date values are determined in accordance with ASC Topic 718. See “Compensation Discussion and Analysis—2019 Long-term Incentive Awards”.
(5) Dr. van Deventer receives his salary in EUR. Amounts were translated to $ using an average exchange rate for the 12-month period ended December 31, 2019 of 1.12 $/euro.
(9) The grant date was January 28, 2016.44
Table of Contents
(10) Mr. Soland served as our Chief Executive Officer from December 2015 until September 2016.OPTION EXERCISES AND STOCK VESTED IN 2019
(11) Dr. Richard’s employment ended effective December 31, 2016.The following table discloses information for each of our named executive officers regarding the exercise of stock option awards and the vesting of certain stock awards as of the end of our 2019 fiscal year.
| | Option Awards | | Stock Awards | |
| | Number of
Shares
Acquired on
Exercise
(#) | | Value Realized
on Exercise
($) | | Number of
Shares
Acquired on
Vesting
(#) | | Value
Realized on
Vesting(1) | |
Matthew Kapusta | | 7,339 | | 459,125 | | 121,022 | | 6,625,222 | |
Robert Gut | | — | | — | | 11,666 | | 534,128 | |
Alexander E. Kuta | | 48,000 | | 2,417,924 | | 2,995 | | 94,702 | |
Sander van Deventer | | — | | — | | 2,598 | | 82,149 | |
(1) Value realized equals number of shares vested multiplied by the closing price of our ordinary shares on the Nasdaq Global Select Market on the day the shares vested.
(12) The grant date was July 13, 2015.
(13) The grant date was January 28, 2016.
(14) Dr. Corzo’s employment ended effective December 31, 2016.
(15) The grant date was May 27, 2014.
(16) The grant date was August 25, 2015.
(17) The grant date was January 28, 2016.
Potential Payments upon Termination or Change of Control
Our employment agreements with our named executive officers provide for payments for such named executive officers upon termination in certain circumstances, including in the event of change in control.
Matthew Kapusta
The Kapusta Employment Agreement requires us to provide compensation and/or other benefits to Mr. Kapusta during his employment and in the event of that executive’s termination of employment under certain circumstances and in the event of termination as a result of a change in control. Those arrangements are described in greater detail below. All severance payments and benefits described below (except for Accrued Benefits) are conditioned upon the execution and delivery to the Company of a General Release of Claims.
Pursuant to the terms of the Kapusta Employment Agreement, iftheir respective employment agreements with the Company, terminates Mr. Kapusta’s employment (or failseach of our named executive officers is or, in the case of Dr. Gut and Dr. van Deventer who have since resigned from the Company pursuant to renew the Kapusta Employment Agreement) withoutSeparation Agreements dated August 25, 2020, was eligible for potential payments and benefits in connection with a termination, including for Cause (defined below) or if Mr. Kapusta resigns or opts not to renew the Kapusta Employment Agreement for Good Reason, (defined below), then Mr. Kapusta is entitled to Accrued Benefits (as defined below), a lump sum equal to one times his then annual base salary and target bonus for the year of termination, accelerated vesting of options and restricted share unit awards which remain unvested as of the termination date, accelerated vesting of performance share unit awards to the extent then earned which remain unvested as of the termination date, and the continuation of certain other benefits.
If Mr. Kapusta’s employmentor in connection with the Company terminates due to his death or disability, he will be entitled to Accrued Benefits and a lump sum bonus payment.
In the event of a Change of Control Termination (as defined below), Mr. Kapusta will be entitled to Accrued Benefits, a paymentControl. The following narrative and tables set forth the potential payments and value of additional benefit that each of our named executive officers would receive in the amount equalscenarios contemplated. The tables below assume that employment terminated and/or the Change of Control occurred on December 31, 2019 and reflect the stock price of the Company on December 31, 2019 of $71.66. Except as otherwise provided, the following definitions apply to the pro rata portion of Mr. Kapusta’s target bonus for the fiscal year in which the termination occurs, an additional lump sum payment equal to two times his then annual base salary and target bonus, accelerated vesting of all equity awards which remain unvested as of the termination date and the continuation of certain other benefits.
If Mr. Kapusta’s employment with the Company is terminated voluntarily without Good Reason by Mr. Kapusta, for Cause by the Company,potential payments upon a vote of the general meeting of the Company’s shareholders to dismiss him or upon a vote of the Board to recommend dismissal from his positions at the Company to the general meeting of the Company’s shareholders and /or to suspend Mr. Kapusta from his positions, then Mr. Kapusta is not entitled to any severance.termination.
“Accrued Benefit” means (a) payment of base salary through the termination date, (b) payment of any bonus for performance periods completed prior to the termination date, (c) any payments or benefits under the Company’s benefit plans that are vested, earned or accrued prior to the termination date (including, without limitation, earned but unused vacation); and (d) payment of unreimbursed business expenses incurred by Mr. Kapusta; and (e) rights to indemnification and directors’ and officers’ liability insurance coverage, under any agreement between the Company and Mr. Kapusta, and/or under any of the Company’s organizational documents.named executive officer.
“Cause” means the good faith determination by the Company, after written notice from the Board to Mr. Kapustathe named executive officer that one or more of the following events has occurred and stating with reasonable specificity the actions that constitute Cause and the specific reasonable cure (related to sections (a) and (h) below): (a) Mr. Kapustathe named executive officer has willfully or repeatedly failed to perform his or her material duties, in his capacity as chief executive officer or as a statutory director, and such failure has not been cured after a period of thirty (30) days’ notice; (b) any reckless or grossly negligent act by Mr. Kapustathe named executive officer having the foreseeable effect of injuring the interest, business or reputation of the Company, or any of its parent, subsidiaries or affiliates in any material respect and which did in fact cause such material injury; (c) Mr. Kapusta’sthe named executive officer’s evidenced use of any illegal drug, or illegal narcotic, or excessive amounts of alcohol (as determined by the Company in its reasonable discretion) on Company property or at a function where Mr. Kapustathe named executive officer is working on behalf of the Company; (d) the indictment on charges or conviction for (or the procedural equivalent or conviction for), or entering of a guilty plea or plea of no contest with respect to a felony; (e) the conviction for (or the procedural equivalent or conviction for), or entering of a guilty plea or plea of no contest with respect to a misdemeanor which, in the Board’s reasonable judgment, involves moral turpitude deceit, dishonesty or fraud, except that, in the event that Mr. Kapustathe named executive officer is indicted on charges for a misdemeanor set forth above, the Board may elect, in its sole discretion, to place Mr. Kapustathe named executive officer on administrative garden leave with continuation of full compensation and benefits under this Agreement during the pendency of the proceedings; (f) conduct by or at the direction of Mr. Kapustathe named executive officer constituting misappropriation or
embezzlement of the property of the Company, or any of its parents or affiliates (other than the occasional, customary and de minimis use of Company property for personal purposes); (g) a breach by Mr. Kapustathe named executive officer of a fiduciary duty owing to the Company, including the misappropriation of (or attempted
45
Table of Contents
misappropriation of) a corporate opportunity or undisclosed self-dealing; (h) a material breach by Mr. Kapustathe named executive officer of any material provision of this Agreement, any of the Company’s written employment policies or Mr. Kapusta’sthe named executive officer’s fiduciary duties to the Company, which breach, if curable, remains uncured for a period of thirty (30) days after receipt by Executivethe named executive officer of written notice of such breach from the Board, which notice shall contain a reasonably specific description of such breach and the specific reasonable cure requested by the Board; and (i) any breach of Section 6 of the Kapusta Employment Agreement.their respective employment agreements.
“Change of Control” means any of the following: (a) any “person,” as such term is used in Sections 13(d) and 14(d) of the Securities Exchange Act of 1934, as amended (the “Act”) (other than the Company, any of its subsidiaries, or any trustee, fiduciary or other person or entity holding securities under any employee benefit plan or trust of the Company or any of its subsidiaries), together with all “affiliates” and “associates” (as such terms are defined in Rule 12b-2 under the Act) of such person, shall become the “beneficial owner” (as such term is defined in Rule 13d-3 under the Act), directly or indirectly, of securities of the Company representing forty (40) percent or more of the combined voting power of the Company’s then outstanding securities having the right to vote in an election of the Board (“Voting Securities”) (in such case other than as a result of an acquisition of securities directly from the Company); or (b) the date a majority of the members of the Board is replaced during any 12-month period by directors whose appointment or election is not endorsed by a majority of the members of the Board before the date of the appointment or election; or (c) the consummation of (1) any consolidation or merger of the Company where the stockholders of the Company, immediately prior to the consolidation or merger, would not, immediately after the consolidation or merger, beneficially own (as such term is defined in Rule 13d-3 under the Act), directly or indirectly, shares representing in the aggregate more than 50 percent of the voting shares of the Company issuing cash or securities in the consolidation or merger (or of its ultimate parent corporation, if any), or (2) any sale or other transfer (in one transaction or a series of transactions contemplated or arranged by any party as a single plan) of all or substantially all of the assets of the Company.
“Change of Control Termination” means (i) any termination by the Company of the named executive officer’s employment other than for Cause that occurs within 12 months after the Change of Control; or (ii) any resignation by the named executive officer for Good Reason that occurs within 12 months after the Change of Control.
“Disability” means an incapacity by accident, illness or other circumstances which renders the named executive officer mentally or physically incapable of performing the duties and services required of him or her on a full-time basis for a period of at least 120 days.
“Good Reason” means that the named executive officer has complied with the Good Reason Process (hereinafter defined) following the occurrence of any of the following events: (a) a material diminution in the named executive officer’s responsibilities, authority or duties (excluding any duties associated with any position that the named executive officer may hold at the Company); (b) a diminution in the named executive officer’s base salary, except for across-the-board salary reductions, based on the Company’s financial performance, similarly affecting all or substantially all other senior management employees of the Company, which reduction does not reduce the named executive officer’s base salary (in the aggregate with any similar reductions during the term of employment) by more than 20% from the named executive officer’s highest base salary; (c) a material change in the geographic location at which the named executive officer provides services to the Company (i.e., outside a radius of fifty (50) miles from their primary business location); or (d) the material breach of their respective employment agreements by the Company (each a “Good Reason Condition”).
“Good Reason Process” means that (a) the named executive officer reasonably determines in good faith that a Good Reason Condition has occurred; (b) the named executive officer notifies the Board in writing of the first occurrence of the Good Reason Condition within sixty (60) days of the first occurrence of such condition; (c) the named executive officer cooperates in good faith with the Company’s efforts, for a period not less than thirty (30) days following such notice (the “Cure Period”), to remedy the Good Reason Condition; (d) notwithstanding such efforts, the Good Reason Condition continues to exist; and (e) the named executive officer terminates the employment within sixty (60) days after the end of the Cure Period. If the Company cures the Good Reason Condition during the Cure Period, Good Reason shall be deemed not to have occurred.
46
Table of Contents
Matthew Kapusta
The following table discloses information about the benefits the named executive officer would receive as of December 31, 2019, at a share price of $ 71.66 upon termination in certain circumstances, including in the event of a change in control.
| | Termination
without Cause
or Resignation
for Good
Reason
($) | | Termination in
Connection
with a Change
in Control
($) | | Death
($) | | Disability(5)
($) | | Retirement(5)
($) | |
Compensation | | | | | | | | | | | |
Cash severance (1) | | 550,000 | | 1,705,000 | | — | | — | | — | |
Pro-rata bonus (1), (2) | | 329,725 | | 302,500 | | 329,725 | | 329,725 | | | |
Long term incentive | | | | | | | | | | | |
Restricted share units — unvested & accelerated | | 4,070,216 | | 4,070,216 | | — | | — | | — | |
Performance share units — unvested & accelerated (3) | | 10,571,411 | | 10,571,411 | | 10,571,411 | | 10,571,411 | | — | |
Stock options —unvested & accelerated | | 4,070,216 | | 4,070,216 | | 4,070,216 | | 4,070,216 | | 4,070,216 | |
Benefits and perquisites | | | | | | | | | | | |
Health insurance (4) | | 24,000 | | 36,000 | | — | | — | | — | |
Total | | 19,615,568 | | 20,755,343 | | 14,971,352 | | 14,971,352 | | 4,070,216 | |
(1) Cash severance and pro-rata bonus are paid as a lump sum, except in the case of base salary paid on termination without cause or for good reason, which is paid over the course of the severance period.
(2) Pro-rata bonus amounts under the “Termination without Cause or Resignation for Good Reason” and “Death” columns are based on actual 2019 annual short-term incentive pay out.
(3) PSU amounts reflect actual earned awards for all completed tranches including the 2019 performance period.
(4) Health costs are based on individual elections and budgeted rates for 2020.
(5) The disclosure assumes the Committee did not exercise its discretion to award pro-rata short-term incentive amounts in the event of disability or retirement.
The Kapusta Employment Agreement requires us to provide compensation and/or other benefits to Mr. Kapusta during his employment and in the event of that executive’s termination of employment under certain circumstances and in the event of termination as a result of a change in control. Those arrangements are described in greater detail below. All severance payments and benefits described below (except for Accrued Benefits (defined below)) are conditioned upon the execution and delivery to the Company of a General Release of Claims.
Other than in the event of a Change of Control Termination (defined below), pursuant to the terms of the Kapusta Employment Agreement, if the Company terminates Mr. Kapusta’s employment (or fails to renew the Kapusta Employment Agreement) without Cause or if Mr. Kapusta resigns or opts not to renew the Kapusta Employment Agreement for Good Reason, then Mr. Kapusta is entitled to Accrued Benefits (defined below), twelve months of base salary, a lump sum bonus payment, accelerated vesting of options and restricted share unit awards which remain unvested as of the termination date, accelerated vesting of performance share unit awards to the extent then earned which remain unvested as of the termination date, and the continuation of certain other benefits.
If Mr. Kapusta’s employment with the Company terminates due to his death or disability, he will be entitled to Accrued Benefits and a lump sum bonus payment.
In the event of a Change of Control Termination (defined below), Mr. Kapusta will be entitled in such circumstances to a lump sum payment equal to two times Mr. Kapusta’s then-current base salary to be paid no later than sixty days after the termination date, his bonus for the year of termination pro-rated based upon Mr. Kapusta’s termination
47
Table of Contents
date, and a lump sum representing and additional two times Mr. Kapusta’s bonus, to be paid no later than sixty days following the termination date.
In the event that Mr. Kapusta incurs excise tax liability pursuant to section 4999 of the Internal Revenue Code, as amended, he will be entitled to certain reductions in his severance payments which will have the result of providing him certain tax relief, all pursuant to the Kapusta Employment Agreement.
If Mr. Kapusta’s employment with the Company is terminated voluntarily without Good Reason by Mr. Kapusta, for Cause by the Company, upon a vote of the general meeting of the Company’s shareholders to dismiss him or upon a vote of the Board to recommend dismissal from his positions at the Company to the general meeting of the Company’s shareholders and /or to suspend Mr. Kapusta from his positions, then Mr. Kapusta is not entitled to any severance.
“Accrued Benefit” means (a) payment of base salary through the termination date, (b) payment of any bonus for performance periods completed prior to the termination date, (c) any payments or benefits under the Company’s benefit plans that are vested, earned or accrued prior to the termination date (including, without limitation, earned but unused vacation); (d) payment of unreimbursed business expenses incurred by Mr. Kapusta; and (e) rights to indemnification and directors’ and officers’ liability insurance coverage, under any agreement between the Company and Mr. Kapusta, and/or under any of the Company’s organizational documents.
“Change of Control Termination” means (a) any termination by the Company of Mr. Kapusta’s employment, other than for Cause, that occurs within the period that starts ninety (90) days preceding the Change of Control and ends on the one-year anniversary of the Change in Control; or (b) any resignation by Mr. Kapusta for Good Reason, that occurs within the period that starts ninety (90) days preceding the Change of Control and ends on the one-year anniversary of the Change in Control.
“Good Reason” means that Mr. Kapusta has complied with the Good Reason Process (hereinafter defined) following the occurrence of any of the following events: (a) a material diminution in Mr. Kapusta’s responsibilities, authority or duties (excluding any duties associated with any position that Mr. Kapusta may hold at the Company); (b) a diminution in Mr. Kapusta’s base salary, except for across-the-board salary reductions, based on the Company’s financial performance, similarly affecting all or substantially all other senior management employees of the Company, which reduction does not occur before January 1, 2016 and does not reduce Mr. Kapusta’s base salary (in the aggregate with any similar reductions during the term of employment) by more than 20% from Mr. Kapusta’s highest base salary; (c) a material change in the geographic location at which Mr. Kapusta provides services to the Company (i.e., outside a radius of fifty (50) miles from Boston, Massachusetts); or (d) the material breachThe foregoing descriptions of the Kapusta Employment Agreement do not purport to be complete and are qualified in their entirety by reference to the full text of such agreement.
Robert Gut
On August 25, 2020, the Company (eachentered into a “Good Reason Condition”separation agreement with Dr. Robert Gut (the “Gut Separation Agreement”) in connection with Dr. Gut’s retirement as an officer of the Company, effective as of October 14, 2020 (the “Gut Separation Date”). “Good Reason Process” shall mean
The Gut Separation Agreement provides that Dr. Gut will receive, subject to the terms thereof, a lump sum severance payment equal to 140% of Dr. Gut’s annual base salary, or approximately $619,549, less applicable taxes and withholdings, within 30 days following the execution of the Gut Separation Agreement and, upon Dr. Gut’s election, reimbursement of full COBRA premiums until the earlier of (a) Mr. Kapusta reasonably determinestwelve (12) months from the Gut Separation Date, (b) the date on which Dr. Gut becomes eligible to participate in another employer’s group health plan, (c) the date on which Dr. Gut (or his eligible dependents, as applicable) is no longer eligible for COBRA coverage or (d) the date on which the Company in good faith determines that payments would result in a Good Reason Condition has occurred; (b) Mr. Kapusta notifiesdiscriminatory health plan pursuant to the Board in writingPatient Protection and Affordable Care Act of 2010. Dr. Gut will also be paid a pro-rata bonus equal to forty percent (40%) of his annual base salary. In addition, Dr. Gut will also be paid for any accrued but unused paid time off as of the first occurrence of the Good Reason Condition within sixty (60) days of the first occurrence of such condition; (c) Mr. Kapusta cooperates in good faith with the Company’s efforts, for a period not less than thirty (30) days following such notice (the “Cure Period”), to remedy the Good Reason Condition; (d) notwithstanding such efforts, the Good Reason Condition continues to exist; and (e) Mr. Kapusta terminates the his employment within sixty (60) days after the end of the Cure Period. If the Company cures the Good Reason Condition during the Cure Period, Good Reason shall be deemed not to have occurred.Gut Separation Date.
Daniel Soland
As described above, Mr Soland resigned from his role as chief executive officer in September 2016. In connection with his resignation, he executed the “Soland Release” which stated he resigned without Good Reason (as defined below) and that he was entitled to no severance or other benefit other than Accrued Benefits (as defined below.)
“Accrued Benefits” means (i) payment of base salary through the termination date, (ii) any payments or benefits under the Company’s benefit plans that are vested, earned or accrued priorPursuant to the termination date (including, without limitation, earned but unused vacation); (iii) payment of unreimbursed business expenses incurred by Mr. Soland;Gut Separation Agreement and (iv) rights to indemnification and directors’ and officers’ liability insurance coverageexcept as providednoted above, Dr. Gut’s participation in the Soland Employment Agreement, under any other agreements between the Company and Mr. Soland, in any insurance policy providing for such coverage or permitting such coverage and/or under anyall of the Company’s organizing documents or the organizing documents of anyemployee benefit plans will terminate as of the Company’s parents, subsidiaries or affiliated entities as applicable.
“Good Reason” means that Mr. Soland has compliedGut Separation Date in accordance with the Good Reason Process (hereinafter defined) followingterms of those plans. Dr. Gut’s grants of equity in the occurrence of anyCompany shall be governed by the terms of the following events: (i) Mr. Soland ceasing to serve as CEO or a memberassociated grant agreements, which are not altered by the terms of the Board; (ii) a diminution in Mr. Soland’s base
salary, except for across-the-board salary reductions, based on the Company’s financial performance, similarly affecting all or substantially all other senior management employees of the Company, which reduction does not occur before January 1, 2017 and does not reduce Mr. Soland’s base salary (in the aggregate with any similar reductions during the employment period) by more than 20% from Mr. Soland’s highest base salary; (iii) a material change in the geographic location at which Mr. Soland provides services to the Company (i.e., outside a radius of fifty (50) miles from Lexington, Massachusetts); (iv) the material breach of this Agreement by the Company or (v) the failure of the shareholders to appoint Mr. Soland to the Board at the EGM (each a “Good Reason Condition”). “Good Reason Process” shall mean that (vi) Mr. Soland reasonably determines in good faith that a Good Reason Condition has occurred; (vii) Mr. Soland notifies the Board in writing of the first occurrence of the Good Reason Condition within sixty (60) days of the first occurrence of such condition; (viii) Mr. Soland cooperates in good faith with the Company’s efforts, for a period not less than thirty (30) days following such notice (the “Cure Period”), to remedy the Good Reason Condition; (ix) notwithstanding such efforts, the Good Reason Condition continues to exist; and (x) Mr. Soland terminates Mr. Soland’s employment within sixty (60) days after the end of the Cure Period. If the Company cures the Good Reason Condition during the Cure Period, Good Reason shall be deemed not to have occurred.
Charles Richard
As described above, Dr. Richard’s employment with the Company ended effective December 31, 2016. In connection with the end of his employment, he entered into the RichardGut Separation Agreement.
The RichardGut Separation Agreement providedalso provides that, through the earlier of the one-year anniversary of the Gut Separation Date or Dr. Gut’s appointment to the Board as a non-executive director, Dr. Gut will provide consulting services to the Company on an as-needed basis relating to the transition of the clinical operations organization. Dr. Gut will be reimbursed at a rate of $500 per hour.
48
Table of Contents
Dr. Gut has also agreed to a customary release and is subject to certain confidentiality, disclosure, non-competition, and non-solicitation restrictions.
The foregoing descriptions of the Gut Separation Agreement do not purport to be complete and are qualified in their entirety by reference to the full text of such agreement.
Alexander E. Kuta
The following table discloses information about the benefits the named executive officer would receive as of December 31, 2019, at a share price of $ 71.66 upon termination in certain circumstances, including in the event of change in control.
| | Termination
without
Cause
($) | | Resignation
for Good
Cause
($) | | Termination in
Connection
with a Change
in Control
(#) | | Death
($) | | Disability(4)
($) | | Retirement(4)
($) | |
Compensation | | | | | | | | | | | | | |
Cash severance (1) | | 573,199 | | 573,199 | | 614,142 | | — | | — | | — | |
Pro-rata bonus(1) | | 179,657 | | 179,657 | | 163,771 | | — | | — | | — | |
Long term incentive | | | | | | | | | | | | | |
Restricted share units — unvested & accelerated | | — | | — | | 1,938,761 | | — | | — | | — | |
Performance share units — unvested & accelerated (1) | | 1,336,029 | | 1,336,029 | | 1,336,029 | | 1,336,029 | | 1,336,029 | | — | |
Stock options —unvested & accelerated | | — | | 24,000 | | 36,000 | | — | | — | | — | |
Total | | 2,112,885 | | 6,721,915 | | 8,697,733 | | 5,945,058 | | 5,945,058 | | 4,609,029 | |
(1) Pro-rata bonus amount under the “Termination without Cause or Resignation for Good Reason” column is based on actual 2019 annual short-term incentive pay-out.
(2) PSU amounts reflect actual earned awards for all completed tranches including the 2019 performance period.
(3) Health costs are based on individual elections and budgeted rates for 2020.
(4) The disclosure assumes the Committee did not exercise its discretion to award pro-rata short-term incentive amounts in the event of disability or retirement.
The Kuta Employment Agreement requires us to provide compensation and/or other benefits to Dr. RichardKuta during his employment and in the event of that executive’s termination of employment under certain circumstances and in the event of termination as a result of a change in control. Those arrangements are described in greater detail below. All severance payments and benefits described below (except for Accrued Benefits) are conditioned upon the execution and delivery to receivethe Company of a General Release of Claims.
Pursuant to the terms of the Kuta Employment Agreement, if Dr. Kuta’s employment is terminated due to the death or Disability of Dr. Kuta, then Dr. Kuta is entitled to Accrued Benefits. If the Company terminates Dr. Kuta’s employment without Cause or if
Dr. Kuta resigns for Good Reason, then Dr. Kuta is entitled to Accrued Benefits, (as defined below) undertwelve months of base salary plus target bonus, a bonus pro-rated to the Richard Employmentdate of termination and based on the target bonus amount set by the Board (currently 40%), and continued coverage through COBRA for a period of 12 months. In the event of a change of control termination then Dr. Kuta is entitled to Accrued Benefits, 18 months of base salary plus target bonus, a bonus pro-rated to the date of termination and based on the target bonus amount set by the Board (currently 40%), and continued coverage through COBRA for a period of 18 months. In the event of a termination of Dr. Kuta’s employment due to death or disability or if Dr. Kuta resigns for Good Reason or upon a Change of Control Termination, Dr. Kuta is entitled to accelerated vesting of options and performance share unit awards that remain unvested as of the termination date. Additionally, if Dr. Kuta retires, he is entitled to accelerated vesting of options. Furthermore in the event of a Change of Control Termination, Dr. Kuta is further entitled to accelerated vesting of
49
Table of Contents
restricted share unit awards, and, to avoid duplication of severance payments, any amount to be paid per the above will be offset by severance amounts paid pursuant to the Company’s change of control guidelines.
Sander van Deventer
On August 25, 2020, the Company entered into a resignation and separation agreement with Dr. van Deventer (the “van Deventer Separation Agreement”) in connection with Dr. van Deventer’s retirement from the Company, effective as of September 14, 2020 (the “van Deventer Separation Date”).
The van Deventer Separation Agreement provides that Dr. van Deventer will receive, subject to the terms thereof, a lump sum separationseverance payment equal €588,700, to compensate for Dr. van Deventer’s loss of $353,109.90, a discretionary bonuswages and/or to supplement benefits paid pursuant to social security legislation. In addition, Dr. van Deventer will also receive his usual gross monthly salary of $103,814€29,435 through the van Deventer Separation Date.
Pursuant to the van Deventer Separation Agreement, all equity incentive awards concluded between the Company and 12,000 PSUs, the vesting of which was acceleratedDr. van Deventer will remain in full force and effect per their terms and will not be altered by the Board.
“Accrued Benefits” meansvan Deventer Separation Agreement, except for the following: (i) all grants of equity will continue to vest until the paymentvan Deventer Separation Date, at which time all such grants of base salary through the termination date, (ii) payment of any bonus for performance periods completed prior to the termination date, (iii) any payments or benefits under the Company’s benefit plans that are vested, earned or accrued prior to the termination date (including, without limitation,equity (other than previously earned but unused vacation),unvested performance share units) shall cease to vest; and (iv) payment(ii) any unvested shares of unreimbursed business expenses.
Deya Corzo
As described above, Dr. Corzo’s employment with the Company ended effective December 31, 2016. In connection withgrants of options to purchase ordinary shares dated on or about September 20, 2017, January 26, 2018, and January 26, 2019, will accelerate and be fully vested as of the end of her employment, she entered into the Corzovan Deventer Separation Agreement.Date.
The Corzovan Deventer Separation Agreement provided for Dr. Corzo to receive Accrued Benefits (as defined below) under the Corzo Employment Agreement,also includes non-competition, non-solicitation and other customary restrictive covenants, as well as a lump sum separation payment of $342,825, a discretionary bonus of $123,588.50 and 22,500 PSUs, the vesting of which was accelerated by the Board..general release.
“Accrued Benefits” means (i)The foregoing descriptions of the payment of base salary through the termination date, (ii) payment of any bonus for performance periods completed priorvan Deventer Separation Agreement do not purport to be complete and are qualified in their entirety by reference to the termination date, (iii) any payments or benefits under the Company’s benefit plans that are vested, earned or accrued prior to the termination date (including, without limitation, earned but unused vacation), and (iv) paymentfull text of unreimbursed business expenses.such agreement.
50
Table of Contents
DIRECTOR COMPENSATION
Overview of Director Compensation Program
Current Director Compensation Arrangements
Our Remuneration Policy provides guidelines forthat our Board may determine compensation paid to non-executive directors. Other than as noted below, our Board has determined that the compensation of non-executive directors. Ourpaid to our non-executive directors are compensatedwill not increase in amount from that paid during the last fiscal year. Our Board-approved non-executive director compensation for their services on our Board is as follows:
· Each non-executive director receivesreceived an annual retainer of $35,000 for a portion of 2019, which increased to $40,000, effective as of the 2019 Annual Meeting, pro-rated for service over the course of the remainder of the year.
· The chairman of the board receives an additional annual retainer of $35,000.$70,000, pro-rated for service over the course of the year.
· Each non-executive director who servicesserves as member of a committee of our Board receives additional compensation as follows:
· Compensation Committee: members receive an annual retainer of $5,000; the chair receives an annual retainer of $10,000.
· Nominating and Corporate Governance Committee: members receive an annual retainer of $5,000; the chair receives an annual retainer of $10,000.
· Audit Committee: members receive an annual retainer of $7,500; the chair receives an annual retainer of $15,000.
· Research and Development Committee: members receive an annual retainer of $5,000; the chair receives an annual retainer of $10,000.
·Each non-executive director receives of an annual equity grant consisting of one-half options and one-half RSUs with a one-year vesting period for each.
The size of the annual equity grant is determined by reference to our peer group companies. In reviewing Board of Director compensation, the Compensation Committee’s independent consultant provides an analysis of cash and equity compensation practices and levels within the same compensation peer group used for the named executive officers. Given the volatile nature of equity prices within our industry, for 2019 it was determined that Directors would receive a fixed value equity award. As a result, the value of the uniQure award will vary relative to our peers who predominantly use fixed share awards, which can vary dramatically in value from year-to-year.
Each annual retainer for Board and committee service is payable semi-annually.
Each member of our Board is also entitled to be reimbursed for reasonable travel and other expenses incurred in connection with attending meetings of the Board and any committee of the Board on which she or he serves.
51
Table of Contents
DIRECTOR COMPENSATION TABLE
The following table summarizes the annual compensation paid to those persons who served as our non-executive directors forduring the fiscal year ended December 31, 2016.2019.
Name | | Fees Earned ($) | | Option Awards
($) | | Restricted Stock
Unit Awards ($) | | Total
($) | | | Fees Earned
($) | | Option Awards
($)(2) | | Restricted Stock
Unit Awards
($)(2) | | Total
($) | |
Philip Astley-Sparke | | 49,781 | | 32,623 | | 34,086 | | 116,490 | | | 85,185 | | 97,725 | | 100,048 | | 280,985 | |
Jack Kaye | | 23,958 | | 12,481 | | — | | 36,439 | | | 57,685 | | 98,077 | | 100,048 | | 255,810 | |
Will Lewis | | 62,500 | | 23,684 | | 34,086 | | 120,270 | | |
David Schaffer | | — | | 14,660 | | 34,086 | | 48,746 | | |
David Schaffer (1) | | | — | | 87,272 | | 100,048 | | 187,320 | |
Paula Soteropoulos | | 42,500 | | 14,660 | | 34,086 | | 91,246 | | | 42,685 | | 87,272 | | 100,048 | | 230,005 | |
Dr. Sander van Deventer | | 45,000 | | 19,172 | | 34,086 | | 98,258 | | |
Madhavan Balachandran | | | 47,685 | | 100,172 | | 100,048 | | 247,905 | |
Jeremy Springhorn | | | 55,185 | | 100,172 | | 100,048 | | 255,405 | |
David Meek | | | 42,685 | | 151,864 | | 95,595 | | 290,144 | |
(1) David Schaffer does not receive cash compensation by agreement due to his relationship with 4DMT.
(2) The value of stock awards and stock options as reported in their respective columns is calculated using the grant date accounting fair value determined in accordance with Accounting Standards Codification 718, Compensation-Stock Compensation (“ASC 718”).
Mr. Kapusta’s and Dr. Gut’s compensation isare disclosed above in the section titled “Management Compensation.”
The following table sets forth information relating to the aggregate number of RSUs and stock options to our Ordinary Shares outstanding at December 31, 2019 for each of our non-executive directors.
Name |
| Award Type |
| Aggregate Number of
Awards Outstanding
(#) |
|
Philip Astley-Sparke | | Option | | 51,685 | |
| | RSU | | 3,230 | |
Jack Kaye | | Option | | 32,685 | |
| | RSU | | 3,230 | |
David Schaeffer | | Option | | 27,685 | |
| | RSU | | 3,230 | |
Paula Soteropoulos | | Option | | 35,685 | |
| | RSU | | 3,230 | |
Madhavan Balachandran | | Option | | 21,685 | |
| | RSU | | 3,230 | |
Jeremy Springhorn | | Option | | 21,685 | |
| | RSU | | 3,230 | |
David Meek | | Option | | 15,295 | |
| | RSU | | 3,230 | |
52
Table of Contents
REPORT OF THE AUDIT COMMITTEE
The report of the Audit Committee is not “soliciting material,” is not deemed “filed” with the SEC and is not to be incorporated by reference into any filing of the Company under the Securities Act of 1933 or the Securities Exchange Act of 1934, both as amended.
The Audit Committee of our Board is responsible for assisting the Board in fulfilling its oversight responsibilities regarding the Company’s financial accounting and reporting processes, system of internal control, audit process, and process for monitoring compliance with laws and regulations.
Management of the Company has the primary responsibility for preparing the Company’s consolidated financial statements, as well as establishing and maintaining the integrity of the Company’s financial reporting process, accounting principles and internal controls. PricewatershouseCoopers AccountsKPMG Accountants N.V., the Company’s independent registered public accounting firm isfor the 2019 financial year, was responsible for performing an audit of the Company’s consolidated financial statements and expressing an opinion as to the conformity of such financial statements with U.S. generally accepted accounting principles.
In this context, the Audit Committee reviewed and discussed the audited financial statements of the Company as of and for the year ended December 31, 20162019 with the Company’s management and PricewatershouseCoopers AccountsKPMG Accountants N.V. To ensure independence, the Audit Committee met separately with PricewatershouseCoopers AccountsKPMG Accountants N.V. and members of the Company’s management. These reviews included discussion with the independent registered public accounting firm of matters required to be discussed pursuant to Statement on Auditing StandardStandards No. 1301, “Communications with Audit Committees,” issued61, as amended (AICPA, Professional Standards, Vol. 1, AU section 380), as adopted by the Public Company Accounting Oversight Board (“PCAOB”). in Rule 3200T. In addition, the Audit Committee received the written disclosures and the letter from the independent registered public accounting firm required by Rule 3526 of the PCAOB requiring independent registered public accounting firms to annually disclose in writing all relationships that, in their professional opinion may reasonably be thought to bear on independence, to confirm their perceived independence and to engage in a discussion of independence, and it has discussed with PricewatershouseCoopers AccountsKPMG Accountants N.V. its independence from the Company.
Based on the reviews and discussions described above, the Audit Committee recommended to the Board the inclusion of the audited financial statements in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2016,2019, for filing with the Securities and Exchange Commission.
The Audit Committee | |
| |
/s/ Jack Kaye | |
Jack Kaye, Chairman | |
| |
/s/ Philip Astley-Sparke | |
Philip Astley-Sparke | |
| |
/s/ Paula SoteropoulosJeremy Springhorn | |
Paula Soteropoulos
| |
| |
August 7, 2017Jeremy Springhorn
| |
53
Table of Contents
GENERAL MATTERS
Availability of Certain Documents
This Proxy Statement a copy of our 2016 Annual Report on Form 10-K and our other filings have been posted on our website athttp://www.uniqure.com/investors-newsroom/sec-filings.php.A copy of our 2019 Dutch statutory annual accounts is available on our website atwww.uniqure.comor may be obtained free of charge by written request.
The original 2016 Report of the Board of Directors, including the 2016 annual accounts, the statement of the external auditors of the Company and the other information as required by Dutch law are available for inspection at the principal executive offices of the Company at the address below as of the date of the notice convening the Extraordinary Meeting.
Please send a written request to investor relations at the Company’s principal executive offices below:
uniQure N.V.
Paasheuvelweg 25a
1105BP
1105 BP Amsterdam
The Netherlands
Attention: Investor Relations
Email: investors@uniQure.com
or to the Company’s administrative offices:
uniQure N.V.
113 Hartwell Avenue
Lexington, MA 02421
United States
Attention: Investor Relations
Shareholder Communications
The Company has a process for shareholders who wish to communicate with the Board. Shareholders who wish to communicate with the Board may write to the Board at the address of the Company’s principal executive office given above. These communications will be received by Investor Relations and will be presented to the Board in the discretion of investor relations. Certain items that are unrelated to the Board’s duties and responsibilities may be excluded, such as spam, junk mail and mass mailings, resumes and other forms of job inquiries, surveys and business solicitations or advertisements. Any communication determined in good faith belief to be frivolous, unduly hostile, threatening, illegal or similarly unsuitable will not be forwarded to the Board.
Proposals for the 20182021 Annual General Meeting of Shareholders
If any shareholder wishes to propose a matter for consideration at our 2018 annual general meeting2021 Annual General Meeting of shareholders, the proposal should be delivered to investor relations at the address above.
To be eligible under the SEC’s shareholder proposal rule (Rule 14a-8(e) of the Exchange Act) for inclusion in our proxy statement and form of proxy for our 2018 annual general meeting2021 Annual General Meeting of shareholders, a proposal must be received by investor relations on or before January 1, 2018,February 19, 2021, unless the date of the 2018 annual general meeting2021 Annual General Meeting is changed by more than 30 days from the date of the 2017 annual general meeting2020 Annual General Meeting of shareholders, and must satisfy the proxy rules promulgated by the SEC.
Any other shareholder proposals and nominations to be presented at our 2018 annual general meeting2021 Annual General Meeting of shareholders, must be received by the Company no later than 60 days before the date of the annual general meeting and must otherwise be given pursuant to the requirements of Dutch law.
Proposals and nominations that are not received by the dates specified above will be considered untimely. In addition, proposals must comply with the laws of the Netherlands, our Articles of Association and the rules and regulations of the SEC.
54
Table of Contents
Other Matters
At the date of the Proxy Statement, management is not aware of any matters to be presented for action at the Extraordinary Meeting other than those described above. However, if any other matters should properly come before the Extraordinary Meeting, it is the intention of
the persons named in the accompanying Proxy Card to vote such Proxy Card in accordance with their judgment on such matters.
August 15, 2017November 2, 2020
| By Order of the Board of Directors,
|
| |
By Order of the Board of Directors, | |
| |
/s/ Matthew Kapusta | |
| |
Matthew Kapusta, Chief Executive Officer, interim Chief Financial Officer | |
and Executive Director | |
55
MMMMMMMMMMMM . MMMMMMMMMMMMMMM C123456789 uniQure N.V. 000004C123456789 000000000.000000 ext 000000000.000000 ext 000000000.000000 ext 000000000.000000 ext 000000000.000000 ext 000000000.000000 ext 000004 ENDORSEMENT_LINE______________ SACKPACK_____________ Electronic Voting Instructions Available 24 hours a day, 7 days a week! InsteadYour vote matters – here’s how to vote! You may vote online or by phone instead of mailing your proxy, you may choose one of the voting methods outlined below to vote your proxy. VALIDATION DETAILS ARE LOCATED BELOW IN THE TITLE BAR. Proxiesthis card. Votes submitted by the Internet or telephoneelectronically must be received by 1:00 a.m., Central Time, on September 14, 2017. MR A SAMPLE DESIGNATION (IF ANY) ADD 1 ADD 2 ADD 3 ADD 4 ADD 5 ADD 6 Votereceived by Internet • Go to www.investorvote.com/November 30, 2020 at 11:59 P.M., Central European Time. Online GIof ntoo welwewct.rinovneicstvoortviontge,.com/QURE • Oror scan delete QR code and control # the QR code with your smartphone • Follow— login details are located in the steps outlined on the secure website Vote by telephone •shaded bar below. Phone Call toll free 1-800-652-VOTE (8683) within the USA, US territories &and Canada on a touch tone telephone • Follow the instructions provided by the recorded messageSave paper, time and money! Sign up for electronic delivery at www.investorvote.com/QURE Using a black ink pen, mark your votes with an X as shown in this example. Please do not write outside the designated areas. q IF YOU HAVE NOT VOTED VIA THE INTERNET OR TELEPHONE, FOLD ALONG THE PERFORATION,VOTING BY MAIL, SIGN, DETACH AND RETURN THE BOTTOM PORTION IN THE ENCLOSED ENVELOPE. q Proposals — The Board recommends a vote FOR Proposals 1 – 2. ForAgainst+ For Against Abstain + 1. Appointment of Jeremy SpringhornRobert Gut, M.D., Ph.D. as a non-executive director. 2. Appointment of Madhavan Balachandran as non-executive director. MMIFMVOTINMG BYMAIL,MYOU MUST COMPLETE SECTIONS A - C ON BOTH SIDES OF THIS CARD. C 1234567890 J N T MR A SAMPLE (THIS AREA IS SET UP TO ACCOMMODATE 140 CHARACTERS) MR A SAMPLE AND MR A SAMPLE AND + MR A SAMPLE AND MR A SAMPLE AND MR A SAMPLE AND 1 U P X3 4 3 8 4 4 1 MR A SAMPLE AND MR A SAMPLE AND MR A SAMPLE AND 02NMFC MMMMMMMMM A Extraordinary Meeting Proxy Card1234 5678 9012 345 X IMPORTANT EXTRAORDINARY MEETING INFORMATION
Table of Contents
. q IF YOU HAVE NOT VOTED VIA THE INTERNET OR TELEPHONE, FOLD ALONG THE PERFORATION, DETACH AND RETURN THE BOTTOM PORTION IN THE ENCLOSED ENVELOPE. q Proxy — uniQure N.V. + 2017 EXTRAORDINARY GENERAL MEETING OF SHAREHOLDERS Proxy and Power of Attorney of Shareholders The undersigned shareholder of uniQure N.V. (the “Company”) hereby constitutes and appoints each of Philip Astley-Sparke and Matthew Kapusta as the attorney and proxy of the undersigned, with full power of substitution and revocation, to vote for and in the name, place and stead of the undersigned at the Extraordinary General Meeting of Shareholders of the Company to be held at Paasheuvelweg 25a, 1105 BP Amsterdam, the Netherlands, at 9:30 a.m. CEST on Thursday, 14 September 2017 and at any adjournments thereof, the number of votes the undersigned would be entitled to cast if present. WHEN PROPERLY EXECUTED, ALL SHARES HELD BY THE UNDERSIGNED SHAREHOLDER WILL BE VOTED IN THE MANNER DIRECTED HEREIN BY THE UNDERSIGNED SHAREHOLDER (UNLESS OTHERWISE INDICATED). IF NO DIRECTION IS MADE, ALL SHARES HELD BY THE UNDERSIGNED SHAREHOLDER WILL BE VOTED FOR EACH OF THE PROPOSALS. (Items to be voted appear on reverse side.) Non-Voting Items Change of Address — Please print new address below. Authorized Signatures — This section must be completed for your vote to be counted. — Date and Sign Below Please sign exactly as name(s) appears hereon. Joint owners should each sign. When signing as attorney, executor, administrator, corporate officer, trustee, guardian, or custodian, please give full title. Date (mm/dd/yyyy) — Please print date below. Signature 1 — Please keep signature within the box. Signature 2 — Please keep signature within the box. MMMMMMM C 1234567890 J N T 8 0 1 8 4 MR A SAMPLE (THIS AREA IS SET UP TO ACCOMMODATE 140 CHARACTERS) MR A SAMPLE AND MR A SAMPLE AND MR A SAMPLE AND MR A SAMPLE AND MR A SAMPLE AND MR A SAMPLE AND MR A SAMPLE AND MR A SAMPLE AND + 1 U P X 4 03C2TC MMMMMMMMM B Authorized Signatures — This section must be completed for your vote to count. Please date and sign below. A Proposals — The BMoaanradgoefmDeinretcBtoarsrdrerceocmommmenedndasvaotveotFeORFOaRll Pthroepnoosmalin1.ees listed, FOR Proposals X – X and for every X YEARS on Proposal X. Extraordinary General Meeting Proxy Card1234 5678 9012 345
You will be able to attend and participate in the Extraordinary General Meeting online and submit your questions prior to and during the meeting by visiting: www.meetingcenter.io/277844738 at the meeting date and time described in the accompanying proxy statement. The password for the meeting is QURE2020. q IF VOTING BY MAIL, YOU MUST COMPLETE SECTIONS A -SIGN, DETACH AND RETURN THE BOTTOM PORTION IN THE ENCLOSED ENVELOPE. q + 2020 EXTRAORDINARY GENERAL MEETING OF SHAREHOLDERS This proxy is solicited by the Board of Directors for use at the Extraordinary General Meeting on December 1, 2020. Proxy and Power of Attorney of Shareholders The undersigned shareholder of uniQure N.V. (the “Company”) hereby constitutes and appoints each of Philip Astley-Sparke, Matthew Kapusta, and David Cerveny as the attorney and proxy of the undersigned, with full power of substitution and revocation, to vote for and in the name, place and stead of the undersigned at the Extraordinary General Meeting of Shareholders of the Company to be held exclusively over the internet via live audio webcast at www.meetingcenter.io/277844738, at 3:00 p.m. CET on Tuesday, December 1, 2020 and at any adjournments thereof, including on any matters that may properly come before the Extraordinary General Meeting, the number of votes the undersigned would be entitled to cast if present. WHEN PROPERLY EXECUTED, THIS PROXY WILL BE VOTED IN THE MANNER DIRECTED HEREIN BY THE UNDERSIGNED SHAREHOLDER. IF NO DIRECTION IS MADE, THIS PROXY WILL BE VOTED FOR PROPOSAL 1. (Items to be voted appear on reverse side) Change of Address — Please print new address below. + C ON BOTH SIDES OF THIS CARD. C BNon-Voting Items Proxy — uniQure N.V. Small steps make an impact. Help the environment by consenting to receive electronic delivery, sign up at www.meetingcenter.io/277844738